Introduction
Intraoperative neurophysiological monitoring (IONM) is a method of real-time evaluation of the functional states of neuronal structures to prevent possible damage. During surgical interventions, the type of anesthetic affects the results of monitoring. In general, total intravenous anesthesia (TIVA) is the gold standard for IONM, because it allows plasma and target-site concentration to be calculated using an method based on age, gender, weight, and height of the patient [1]. However, TIVA is often costly, requires a infusion pump, and may not be rapidly titratable. Moreover, it may induce a respectable reduction in the cerebral blood volume [2], and affect the hemodynamic and cardiovascular parameters [3]. Inhalational anesthetics have a smaller impact on several parameters than do intravenous anesthetics. However, they increase the latency and reduce the amplitude [4]. Herein, we report four patients who underwent inhalational anesthesia because of cardiovascular disease, with IONM during the operation.
Cases
Four patients (2 men and 2 women; mean age, 69.25 years [range 63-76 years]) underwent inhalational anesthesia with intraoperative monitoring from March, 2016 to March, 2017 (Table 1). In one patient, intracranial surgery performed, and the other three patients underwent spinal surgeries. Because of underlying diseases or unstable hemodynamic conditions, all patients received inhalational anesthesia. Three patients were premedicated with propofol, and one patient with midazolam before induction of anesthesia. The induction drug was sevoflurane (3 cases) or desflurane (1 case). Among 4 cases, 2 cases got surgery with 0.5 minimum alveolar concentration (MAC) of inhalational anesthesia and other 2 with 1.0 MAC. To evaluate sufficiency of neuromuscular block, we did train of four (TOF) method. In abductor pollicis brevis mulscles, two of four responses to median nerve stimulation would be considered enough for motor evoked potentials (MEP) monitoring [5].
The somatosensory evoked potentials (SSEPs) and MEPs were recorded in the patients. For MEP monitoring, transcranial electrical motor constant-voltage stimulation was performed with a pair of subdermal electrodes placed at the C3/C4 positions, which were 5 cm from the Cz position. Myogenic MEPs recorded bilaterally in the upper and lower limb muscles (abductor pollicis brevis, abductor digiti minimi, tibialis anterior, and abductor halluces). Transcranial electrical stimulation was performed in 5 successive 400-V intensity applications with 0.5-1.0-ms duration and 2-ms inter-stimulation interval. The SSEP also performed during IONM. Subdermal electrodes for stimulation were placed on the median nerve of the wrist and posterior tibial nerve of the ankle bilaterally. Stimulation intensity was 20-30 mA with a duration of 200 ms. Recording electrodes were located at Fz, Cz, C3, and C4 according to the international 10-20 system. The averaged waveform was obtained with stimulation during surgery.
IONM was started after anesthesia and ended when all procedures were done. Before initiation of the surgical procedures(laminectomy or craniectomy), baseline MEP and SSEP waveforms were obtained. We compared these waveforms to the waveforms obtained afterward. Warning criteria for notification was defined as a decrease of more than 50% of MEP or SSEP amplitude compared to the baseline data. After all surgical procedures were done, we checked recovery of MEPs and SSEPs.
Using what we learned from these cases, we can expound our experiences using IONM with inhalational anesthesia according to the MAC level.
A 65-year-old woman visited our hospital for dizziness, and we detected an asymptomatic unruptured aneurysm (3.6 × 1.8 mm) in the right anterior communicating artery. Surgical clipping was performed with IONM. Due to her underlying medical condition (hypertension) and frequent ventricular premature contractions (15 times/min), as detected on the electrocardiogram, we decided to use inhalational anesthesia with sevoflurane instead of TIVA. TOF monitoring was 2/4. Osteoplastic craniotomy and aneurysmal neck clipping were performed. MAC of sevoflurane was 0.5. SSEP and MEP were monitored during surgery. MEP transcranial electrical stimulation performed according to routine protocol. Myogenic MEP response were ranged from 31.8 to 657 µV. It was relatively small amplitude than myogenic MEP response with TIVA. But we could get reliable MEP wave form during surgery.
The aneurysm was clipped, and the amplitude of SSEPs and MEPs remained at to control level (Fig. 1-A). Microvascular Doppler ultrasonography recording, with a 1-mm tipped, 16-Hz Doppler probe, was also performed. Through microvascular Doppler ultrasonography, we obtained baseline local flow around the aneurysm. After aneurysm clipping, we ascertained the patency of the clipped vessel and identified no vasospasm or occlusion. Perioperative modified Rankin Scale (mRS) was not changed (pre-operative state: mRS0, post-operative state: mRS0).
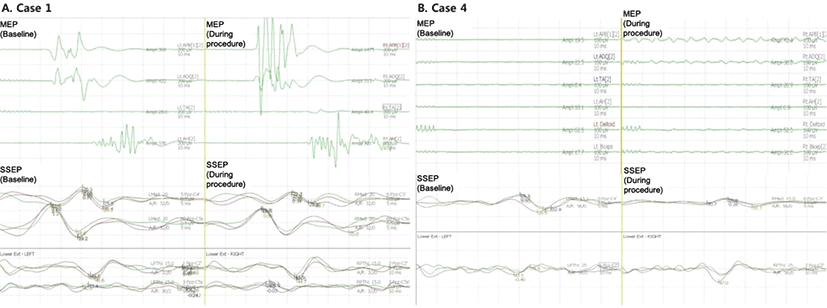
A 74-year-old-man complained of paresthesia in both arms. A spine MRI showed stenosis of the cervical spinal cord (level C4 to C6). Because of his old age and unstable blood pressure preoperatively, cervical spinal fusion was performed under inhalational anesthesia using sevoflurane (TOF at 2/4). Because the depth of anesthesia was insufficient, MAC was raised from 0.5 to 1. We also followed routine protocol for SSEP and MEP stimulation. The amplitude of SSEP wave form was diminished but recognizable. However, there was no detectable myogenic response in the MEP with 1.0 MAC (Fig. 1-B).
Discussion
In the above-mentioned 4 cases, 3 received inhalational anesthesia with sevoflurane and 1 with desflurane for their surgeries. There was no worsening observed on the MRS after the operations. Compared to the cases with 0.5 MAC, the cases with 1.0 MAC showed incomplete IONM results. We could recognize MEPs and SSEPs changes during surgery with 0.5 MAC. When we maintain a total MAC of 0.5, we can monitor reliable MEPs and SSEPs (Table 1). Hence, it might be possible to monitor IONM under inhalational anesthesia with an MAC of 0.5.
Inhalational anesthetics inhibit the pyramidal activation of spinal motor neurons at the anterior grey column or depress the synaptic transmission in the cerebral cortex. Thus, they abolish MEPs more easily than SSEPs. At an MAC value of 1.0, MEP readings are unreliable [4]. Lo et al. reported 10 scoliosis cases which showed successful acquisition of MEPs under desflurane anesthesia with an MAC of 0.5 [6]. Chong et al. found that transcranial multi-pulse stimulus allows reproducible MEP monitoring up to an MAC of 0.7 with both desflurane and sevoflurane [7].
According to Husain, SSEPs were known to be resistant to inhalational anesthesia [8]. But in our case, the amplitude of SSEPs in case 3 and 4 were decreased. Even we could obtain reliable SSEP waveform during surgery, inhalational anesthetic agents also could affect SSEP monitoring.
When considering our data and previous research, it is important to modulate the concentration of inhalational anesthesia during IONM. In the case with an unstable hemodynamic state and contraindication to intravenous anesthesia(e.g. propofol infusion syndrome), inhalational anesthesia would be considered as the choice of anesthetic method. In these cases, inhalational anesthesia could be performed with an MAC of 0.5.
In this study, there are several limitations. First, mentioned above, inhalational anesthetic drugs suppress cortical activities and affect intraoperative monitoring waves [4]. Like one of our cases (case 2, Table 1), sometimes it would be difficult to recognize warning criteria due to small waveform. Also, IONM would be difficult at an MAC of 0.5 if the depth of anesthesia is insufficient. MAC of an inhaled anesthetic is the alveolar concentration that prevents movement in 50% of patients in response to a standardized stimulus like surgical incision [9]. It is random values determined by various factors (e.g. age, blood pressure, anemia, thyroid function, body temperature) and can be set according to clinical situations. In case 4, surgical procedure was started at MAC of 0.5 but MAC was raised to 1.0 because of insufficiency of anesthesia. This change could affect IONM and lead to suppression of monitoring modalities. In last, electroencephalogram (EEG) was not performed on an aneur ysm clipping case (Case 1). EEG would provide more informations about effects of inhalational anesthesia during brain surgery [10].
In conclusion, although it can be applied limited patients, we should alert to waveform changes and MAC value when using inhalational anesthesia in IONM.
