Introduction
Along with brain and spine surgeries which use intraoperative neurophysiological monitoring (INM) in most cases, peripheral nerve surgeries can also utilize INM. Here, we demonstrate a brachial plexus tumor surgery case with significant INM changes during the surgery and permanent neurological deficits. This study illustrates the usefulness of INM during the brachial plexus tumor surgery.
Case presentation
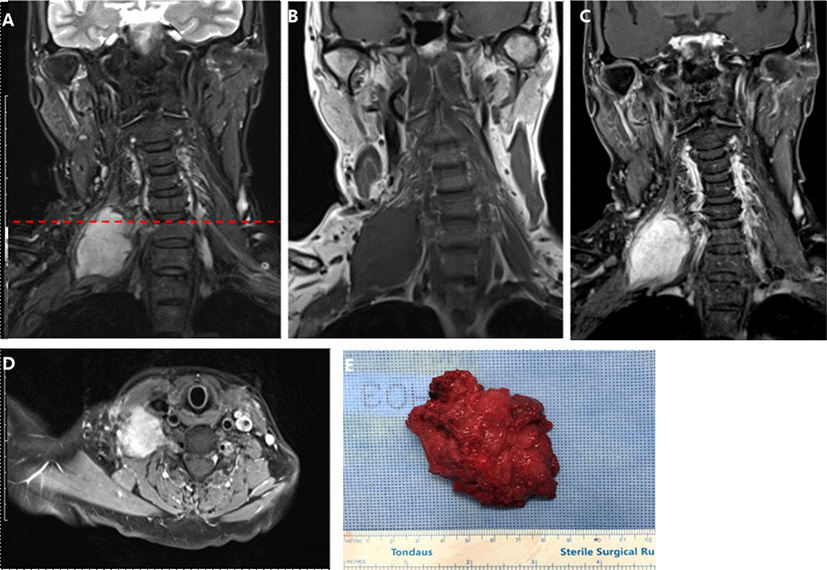
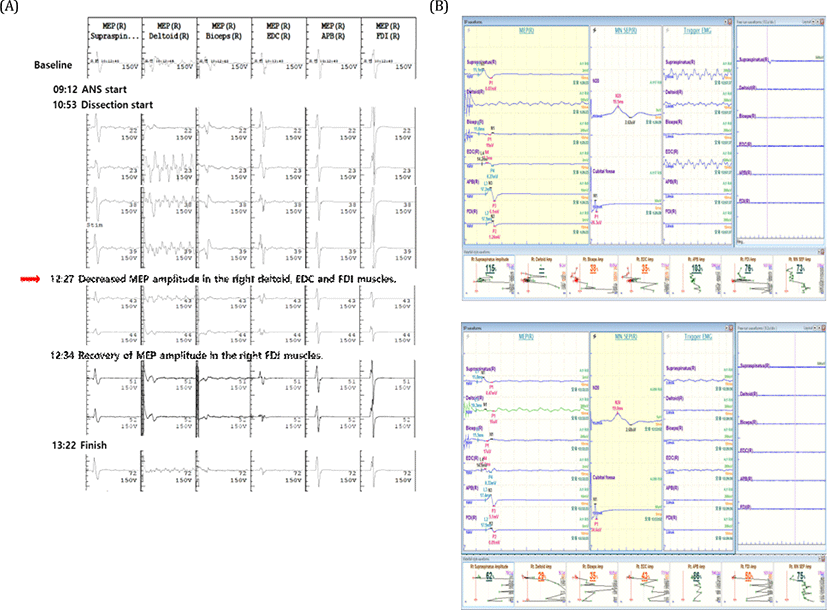
A 67-year-old woman had been experiencing palpable mass on the right supraclavicular area with pain radiating to the ipsilateral arm and subscapular area for one and a half years. After several months, she started to complain of dyspnea at rest. At another hospital, her MRI study revealed schwannoma in the right brachial plexus and chest X-ray showed flattened and elevated right diaphragm. She received partial resection of the tumor without neurophysiologic monitoring (INM). Postoperative power of right proximal upper extremity became grade 2 which recovered several days after the surgery. She also developed loss of pain and temperature sensation in the territory of spinal root level C2-T3 after the surgery, which took more than 6 months to recover. Because the cervical mass became more massive with aggravating pain, she visited our hospital and agreed to receive total resection of the tumor. Preoperative neurological examination showed hyperesthesia and hyperalgia on her right arm involving ipsilateral supraclavicular nerve (C3-4), axillary superior lateral cutaneous nerve (C5-6), radial posterior branch cutaneous nerve, inferior lateral antebrachial cutaneous nerve, posterior antebrachial cutaneous nerve (C5-7), lateral antebrachial cutaneous branch of musculocutaneous nerve (C5-6), and proper palmar and palmar digital branch of median nerve (C5-8). The power of right shoulder abduction was grade 4+. Deep tendon reflex (DTR) was normoactive without any pathological reflexes. Follow-up MRI revealed increased size of the mass (3.3 × 3.8 × 4.2 cm → 3.7 × 4.1 × 4.6 cm). The presurgical diagnosis was neurogenic tumor arising from the right brachial plexus based on the MRI findings (Fig. 1. A-D). General anesthesia included intravenous infusion of propofol (2.0-3.0 mcg/ml) and remifentanyl (5-9 ng/ml). The short-acting neuromuscular blocker, rocuronium (30 mg) was injected only for the endotracheal intubation. For INM, we measured triggered/free-running EMG, and MEP on right supraspinatus, deltoid, trapezius, biceps brachii (BB), extensor digitorum communis (EDC), flexor digitorum interossei (FDI) muscle, and abductor pollicis brevis (APB). We also monitored median nerve somatosensory evoked potential (SSEP). We used MEE-1000 IOM/EP intraoperative monitoring system (Nihon Kohden©, Tokyo, Japan). During the operation, we alarmed surgeons upon our alarm criteria: decrement more than 50% of baseline amplitude of MEP/SSEP or tonic EMG activity. During the surgery, severe fibrosis and adhesion was present around the tumor, which compressed right phrenic nerve at its left lateral margin and invaded into the adjacent muscles and connective tissues. Triggered EMG was utilized to avoid cutting or coagulating neural structures. During the dissection of inferior margin of tumor, the surgeon had to sacrifice a superior trunk of brachial plexus innervating right deltoid, EDC and biceps muscle confirmed by triggered EMG. The surgeon completely removed 8-cm-sized tumor (Fig. 1-E). After sacrificing superior trunk of brachial plexus, first decrement of the MEP amplitude in the right deltoid (29 %), right biceps (29%), right EDC (43%), right FDI (50%) were found (Fig. 2). The final MEP findings at the end of surgery were as following: complete absence of MEP at right deltoid muscle, significant amplitude decrement of MEP at right biceps and EDC (38% and 35%, respectively), and recovery of MEP at right FDI muscle. The amplitude of SSEP in the right median nerve did not change significantly during the operation. During the operation, there was no significant change in the blood pressure and body temperature, and no adverse events such as hypoxia or massive bleeding. The histopathological findings suggested desmoid type fibromatosis (DF) in the right brachial plexus. Postoperative neurological examination showed marked motor and sensory deficit in the right upper extremity. Motor examination revealed power deficit in the shoulder abduction (0/5), shoulder adduction (1/5), elbow flexion (0/5), elbow extension (1/5), wrist flexion (3/5), wrist extension (2/5), finger spreading (3/5), and thumb abduction (2/5). The pin-prick and temperature sense was 70% of baseline in the superior lateral cutaneous branch of axillary nerve (C5-6), 40% in the radial nerve area (C5-7), 70% in the median nerve area (C8-T1), and 70% in the ulnar nerve area (C8-T1). Vibration sense was severely decreased and position sense was mildly decreased in the right upper extremity. On the POD #8, postoperative NCV and EMG study suggested an early brachial pan-plexopathy, predominantly in the superior brachial plexus (Table 1).
Motor nerve conduction study was tested in the bilateral median, ulnar, radial, musculocutaneous, axillary and suprascapular nerves. The results show unobtainable CMAPs in the right musculocutaneous and axillary nerves, and relatively decreased amplitude of CMAPs in the tested nerves of right side in compared to the left side. The terminal latency and motor conduction velocity in the right radial, ulnar and median nerves were in normal range. ND: not detected; CMAP: compound muscle action potential.
Sensory nerve conduction study was tested in the bilateral median, ulnar, radial, medial antebrachial cutaneous and lateral antebrachial cutaneous nerves. The conduction velocities in the tested nerves were in normal range but SNAP amplitudes in the right side were relatively decreased than to the left side. SANP: sensory nerve action potential.
Electromyography (EMG) was done in the right C5-T1 paraspinal, deltoid (Del), rhomboid, biceps brachii (BB), triceps brachii (TB), pronator teres (PT), abductor pollicis brevis (APB), and first dorsal interosseous (FDI) muscles. The results show increased insertional activities in the right FDI, APB, PT, BB, TB, and Del muscles. On the rest, there were positive sharp waves in the right FDI, APB, PT, BB, TB, and Del muscles. On mild contraction, there was polyphasic high amplitude and long duration muscle unit action potentials (MUAPs) in the right APB and FDI muscles. On maximal contraction, reduced interference patterns were observed in the right FDI, APB, PT, BB, TB, and Del muscles. Fibs: fibrillation potentials; PSW: positive sharp waves; Dur: duration; Amp: amplitude; Poly: polyphasic; Recrt: recruitment; Int Pat: interference pattern; FDI: flexor digitorum interossei.
Discussion
DF is a rare, monoclonal fibroblastic proliferative disease which is characterized by infiltrative growth to adjacent tissues and high incidence of local recurrence even after gross total resection of tumor. Supraclavicular lesion is relatively common site of extra-abdominal presentation of DF which comprising 9%-35% of cases [1,2]. However, DF only accounts for less than 4% in the primary brachial plexus tumor [3,4]. Although there is no consensus on the treatment, watchful observation is considered in the asymptomatic patients [5], but surgical resection is usually required in the case of functional loss derived from tumor invasion. Gross total resection with negative tumor margin and preserving neural structures are recommended along with adjuvant radiotherapy, hormone therapy (e.g., antiestrogen), and chemotherapy which can improve long-term clinical outcome by reducing local recurrence rate [6,7].
Despite of the unclear tumor margin due to its adhesions and invasion to adjacent tissues, the surgeon could make important surgical decision upon information provided by INM, which delineated neural structures using triggered EMG and monitored neural integrity throughout the surgery using MEPs and free-running EMG. In this patient, right C5-6 nerve roots and right superior trunk were embedded in the tumor and sacrificed. Intraoperative findings were confirmed by postoperative neurophysiologic examination and EMG & NCS. However, the postoperative sensory deficit on the radial nerve innervated area could not be predicted with SSEP. This patient might have been better monitored by radial nerve SSEP rather than median nerve SSEP although radial nerve SSEP is not routinely used in other types of surgeries.
The anatomical complexity and tumor infiltration make technical difficulty in the plexus surgery, sometimes resulting in inevitable postsurgical neurologic complications. Carefully planned INM can provide valuable information during the surgery to avoid neural damages.
