Introduction
Performing cortical and subcortical motor evoked potentials (MEPs) mapping by electrostimulation during resection of primary brain tumors (PBT) decreases the postoperative motor deficits [1]. In asleep craniotomies, cortical motor function is usually mapped with a train-of-five (5 pulses, with 500 ms and 250–500 Hz frequency) delivering anodal current through a probe with the return electrode placed on forehead. However, at subcortical level, cathodal current is applied through the probe due to corticospinal tract fibers increased sensitivity when compared to anodal current [2].
The physiological organization of cortical cells within the primary motor gyrus is essentially contributing to the configuration of cortical maps [3]. However, after a brain insult such as the one related to PBT development, molecular changes, cellular death, reorganization and regeneration processes are expected to occur [4]. During such remodelling, current input selectivity may be altered in the corticospinal tract and the influence of descending inputs on the motor tract electrical proprieties may change. We describe a case of a right PBT recurrence and discuss possible explanations for the surprising findings related to electrical current sensitivity.
Clinical case
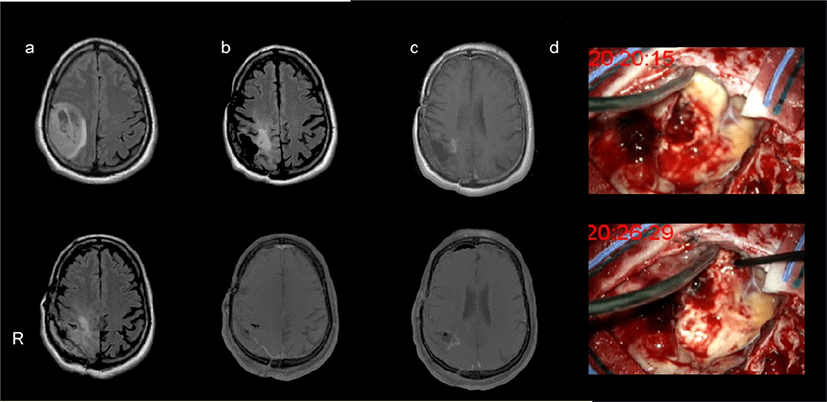
A 54-year old male with a history of a right parieto- frontal anaplastic astrocytoma (Grade III - 2010 WHO classification), was submitted to gross total resection at the Hospital Garcia de Orta, E.P.E. in 2012 without intraoperative neurophysiological neuromonitoring. Surgical treatment was followed by radiotherapy (60 Gy in 2 Gy fractions) and 6-month chemotherapy with Temozolomide. The patient showed no motor deficit after treatment. Eight years later, he presented with left arm focal motor seizures due to PBT recurrence, characterized by a new contrasting lesion in the postgadolinium T1 magnetic resonance imaging sequence, accompanied by vasogenic oedema. Scans are demonstrated in Fig. 1. At the time, seizures were controlled with 2,000 mg Valproic Acid and glioma tumor recurrence was managed with further Temozolomide treatment. Despite the chemotherapy, follow- up imaging further suggested tumor recurrence two years later. Within this period, no new motor deficits or seizures were noted. The patient was admitted for an asleep craniotomy by the same surgeon that performed the previous resection.
Anaesthetic induction was achieved using a bolus of muscle relaxant for intubation purposes and maintained with total continuous infusion of propofol (2 mg/kg/min) and remifentanil (1.5 mg/kg/min). Intraoperative neurophysiological neuromonitoring with direct cortical and subcortical stimulation (OSIRIX Inomed Medizintechnik, Germany) were used for motor function mapping and monitoring. A 6-contact strip electrode (with 10 mm diameter electrodes spaced by 1 mm) was placed over the motor gyri, and a 1.6 mm diameter monopolar probe combined with an aspiration system, delivered 300 Hz train-of-five pulses (500 msec each at 1–30 mA) with the return electrode on the forehead. MEPs were recorded in the contralateral mentalis, biceps brachii, extensor digitorum communis, abductor pollicis brevis (APB), tibialis anterior and abductor hallucis muscles using twisted paired 13 mm or 18 mm subdermal needles with 0.5 to 2,000 Hz signal filtering and 128 kHz sampling rate. The strip electrodes were used to perform electrocorticography to detect epileptic seizures before, and after electrostimulation.
After craniotomy, right precentral gyrus and tumor cavity were exposed (Fig. 1) Motor gyri was mapped using somatosensory evoked potentials to stimulation of the contralateral median nerve [5]. The strip electrode above N20 phase reversal was selected to stimulate the cortex and assess motor function. After N20 phase reversal, the cortical site beneath the strip electrode over the motor gyri was mapped using an handheld. Motor function probe mapping was made around the cavity boundaries with anodal current for anatomical cortical sites, and cathodal current for subcortical sites, at an intensity of 25 mA. The APB was selected as the target muscle. We searched for muscle ‘hotspots’, as the sites around the expected motor cortex area corresponding to the APB from where the largest MEPs could be obtained using lowest stimulus intensity. These were determined for cortical level using anodal current and for subcortical level using cathodal current. Due to the hand-held probe variable aligning with the brain surface, five MEPs were recorded from such sites, and their mean latency and amplitude were measured. Then, we looked for the motor threshold in the same sites. The intensity threshold was defined as the lowest intensity with which we could obtain 5 motor responses greater than 50 μV out of 10 stimuli in the APB. The stimulation protocol was repeated after tumor resection.
The mean of five APB MEPs parameters are summarized in Table 1. Cortical site was localized over the precentral gyri, closely related with strip electrode placed at N20 evoked potential phase reversal. Subcortical mapping site was anatomically 15 mm beneath the cortical APB hotspot with not macroscopically recognized sulci (Fig. 1). The lowest intensity used to evoke the greatest APB MEP amplitude was 22 mA for cortical site and 23 mA for subcortical site. After tumor resection, an APB MEP of higher amplitude than before the operation was recorded from stimuli applied to the cortical site, the MEP amplitude was larger with anodal than with cathodal current (Table 1, non-parametric Mann-Whitney test, p = 0.019). Regarding the APB MEP latencies, those obtained after cathodal current were shorter when compared to anodal current, although the differences were not statistically significant (p = 0.073). Cortical and subcortical thresholds after resection were lower than at the beginning of the surgery (Table 1). At the site of subcortical stimulation, the threshold was lower with anodal than with cathodal current (p = 0.049). Neither after-discharge nor seizures were identified after brain mapping. Postoperative neurological examination was carried out 72 hours after surgery showing hyperalgic hypoanaesthesia of the left upper limb without motor deficit. At one month follow-up, there were no signs of tumor recurrence. The patient maintained the sensory disorder.
Discussion
This patient showed a low subcortical motor threshold when using anodal current after PBT recurrence. Such brain electrostimulation property is a rare finding and more so after prolonged disease duration in a patient submitted to various treatments. This observation, in a patient previously treated with surgical resection and adjuvant radio-chemotherapy, may lead to further physiological understanding of reorganizational changes of the motor pathway after brain surgery.
In humans, pyramidal fiber axon cells represent about 20% of the fifth layer of the primary motor cortex [6]. Cortical stimulation activates the corticospinal tract inducing a direct wave (D wave) and various indirect waves (I waves) that can be recorded with epidural electrodes. Anodal cortical stimulation is, in general, more effective than cathodal [7]. Anodal current enters the cortical layer and hyperpolarizes dendrites, causing a relative depolarization in the axons with lower threshold. Differently, subcortical pyramidal tract axons are more responsive to cathodal than to anodal current stimulation [1,7]. This is believed to occur in part due to a better axonal response to cathodal stimulation and axon arrangement in relation to the probe. Low motor threshold is achieved with a perpendicular spatial relation between the probe and the corticospinal tract. In such case, the electrical current enters perpendicular to the axons. In fact, the current spreading in a radial fashion allows to work on thresholds and to understand the distance from the probe to the corticospinal tracts [3,8].
In our case, a motor threshold about 4 mA lower was obtained with anodal than with cathodal current when stimulating subcortically. In addition, at the same site APB MEP latency was shorter when using cathodal current, which may suggest a more distal tract activation. In the first surgery (i.e. eight years before), motor mapping was not used and, therefore, we are unable to have a within-patient control condition. Nonetheless, this finding is rare even in the presence of tumors, and maybe due to technical or physiological aspects. Motor threshold varies according to the spatial relation between the probe and the depolarized axons. We did our best to search for the optimal site where the lower motor threshold could be obtained from, by mapping the cortical and subcortical surface available. Having had a previous resection, the stimulated area may also not have been optimal despite the appropriate display of the precentral gyrus and tumor cavity. The absence of parenchyma within the tumor cavity may have also decreased the resistance to current dispersion, implying a pyramidal cell activation more closely to the cell bodies than to the axons [7]. However, some findings suggested an adequate technical motor function mapping. Central sulcus was identified by N20 phase reversal. Anodal current threshold was lower than cathodal current at cortical site, and decreased after tumor resection which in a patient without motor deficit may suggest a low mass effect of tumor on corticospinal tract. In fact, the APB MEP amplitude increased significantly after tumor resection. The ME data recorded are prone to unreliable statistical comparisons, misleading a false high subcortical MEP threshold, which warrants for a large dataset corroboration and pathological evidence.
After cortical tumor resection, parenchyma organization may not be macroscopically maintained, particularly after tissue radiotherapy [1,8,9]. This may trigger delayed brain injury, characterized by demyelination and vascular and white matter abnormalities [1,8]. Such event, with malignant cell proliferation, should evoke molecular remodelling with the destruction of astrocytes by macrophage invasion and cell death [5, 9]. However, in this case, the absence of motor deficit at the time of the initial diagnosis and recurrence suggests an intact corticospinal tract with no impairment of motor axon function. On the contrary, the remodeling of the white matter fibers around the motor tract, induced by the treatments (surgery and radio-chemotherapy) and the period of time until the recurrence surgery (8 years in total), may explain the spatial rearrangement which made neuron cell bodies more prone than the axons to receive the inputs generated by the electrical stimulation with anodal current. In fact, subcortical APB MEP showed an increased latency with anodal current compared to cathodal current stimulation, which may suggest a more proximal activation site of the corticospinal tract. However, other hypothesis may explain such low threshold using anodal current, such as the current spread to the motor gyri using a reasonable high mapping intensity. Apart from leading to these physical/physiological hypotheses, our results have a practical side: testing both, cathodal and anodal current stimulation leads may be advantageous for a better motor threshold definition during human brain mapping near extensive remodelled cortices after treatment.
