Introduction
Recent advances in surgical techniques in the robotic radical prostatectomy emphasize the method of neurovascular bundle (NVB)-sparing to save erectile function [1]. Erectile dysfunction (ED) depends on autonomic nervous system (ANS) of which electrical signal is difficult to record consistently. However, corpus cavernosum electromyography (CC-EMG) can directly measure the neuro-electrical activity of the corpus cavernosum (CC), suggested as a potential diagnostic tool of neurogenic ED since 1989 [2]. Here, we present a case using intra-operative nerve monitoring and mapping (IONM) of the cavernous nerve through CC-EMG during robot-assisted radical prostatectomy.
Case presentation
A 65-year-old male had been diagnosed of prostate cancer and underwent robot-assisted laparoscopic radical prostatectomy. The pathologic stage was T2CN0 confirming negative surgical margin without further pelvic lymph node dissection.
General anesthesia was induced by total intravenous anesthesia including continuous infusion of propofol and remifentanil of which initial concentrations were 4 μg/mL and 4 ng/mL respectively. The following effect-site target-controlled infusion was maintained within a bispectral index of 30–60. Controlled muscle relaxation using rocuronium at a dose of 0.1–0.2 mg/kg/h was administered only for intubation and afterwards a train-of-four (TOF) responses to be within 5% variation and kept as TOF count of 2 especially for during CC-EMG monitoring while all other operative manipulations were stopped to prevent any possible interference or artifact.
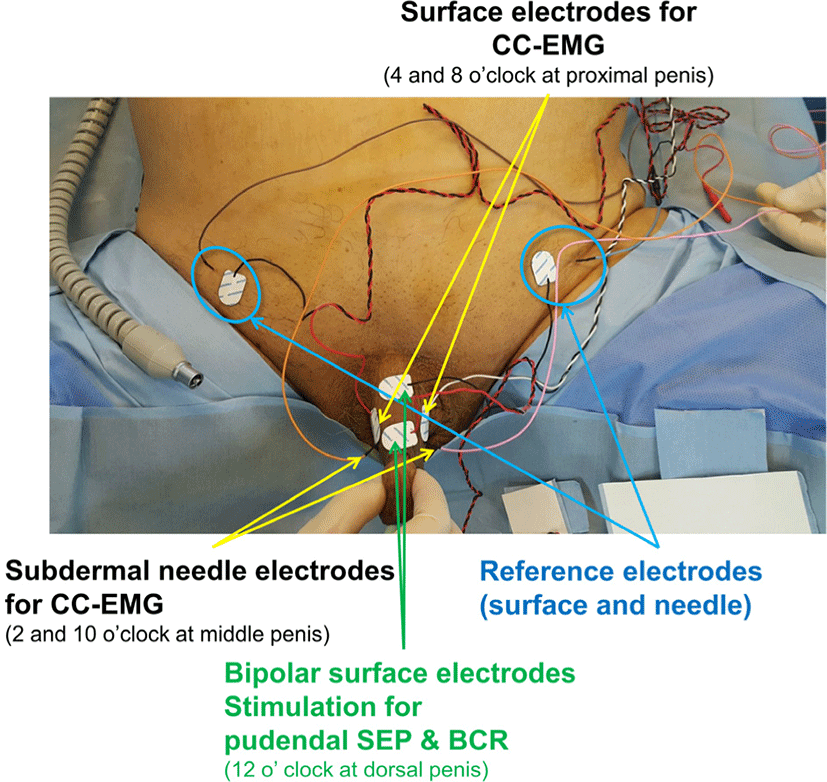
Recording surface electrodes were placed on the proximal penile shaft at lateral ventral sides (at 4 and 8 o’clock positions in the proximal penile shaft) and another set of intracavernous subdermal needle electrodes were placed lateral dorsal sides (at 2 and 10 o’clock positions in the middle penile shaft). Reference electrodes were placed in the ipsilateral suprapubic area respectively [3]. Pudendal somatosensory evoked potential (SEP) and bulbocavernosus reflex (BCR) were also monitored for S2–4 levels concurrently, using surface electrodes placed midline at dorsal sides for stimulation and recording at subdermal needle electrodes at the scalp (Cz-Fz montage) for pudendal SEP and external anal sphincter muscles (at 3 and 9 o’clock positions) for BCR which did not change during operation of 320 minutes. All placed stimulation, recording, and reference electrodes were illustrated in Fig. 1.
Just before NVB dissection and immediately after prostate removal, spontaneous CC-EMG were measured for 1–3 minutes with filtering of 1–50 Hz (Fig. 2-A). All operative manipulations and movements were withhold during spontaneous CC-EMG recording to capture spontaneous activity in the corpora smooth muscles. Both surface and needle electrodes showed low-frequency, highly polyphasic continuous waveforms. Although it was known to take 30–45 minutes to capture spontaneous CC-EMG in a laboratory room [4], this case reported signals within 3 minutes. The electric source of spontaneous CC-EMG is regarded as a continuous sympathetic suppression signal, which ceased during tumescence and full erection [2].
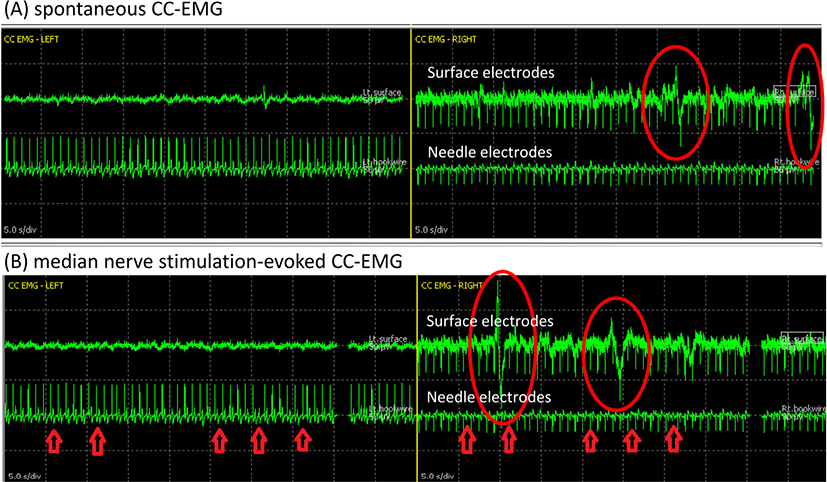
Median nerve stimulation-evoked CC-EMG (MNSE CC-EMG) was monitored as the baseline test at just before trocar insertion and as the final test after drain insertion (Fig. 2-B). Monophasic orthodromic stimulation at the left wrist with intensity of 30 mA and duration of 100 ms was employed to evoke systemic, sympathetic responses, which can also be induced by startling stimulus such as sudden noise or a deep breath because it shares the same principles with the sympathetic skin response test. This evoked cavernous activity is a measure of the autonomic innervation of CC which become absent in those with autonomic neuropathy [5]. Spontaneous and evoked CC-EMG complexes were reported to show the same morphology [4], and our case during IONM also did not show any distinction between spontaneous and MNSE CC-EMG regarding waveform morphology of polyphasic, low-frequency waves beginning with an upward deflection.
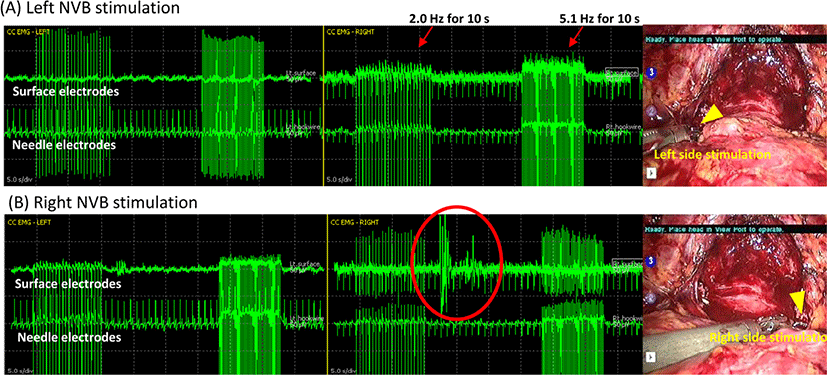
The NVB-triggered CC-EMG used da Vinci Maryland robotic bipolar forceps (Intuitive Surgical, Sunnyvale, CA, USA), connected to a customized bipolar electrical cable to an IONM system (NIM-Eclipse, Medtronic Xomed, Jacksonville, FL, USA). This grasped and conveyed electric signals to the proximal NVB bilaterally at the prostate base level with a single-pulse train stimulation of 2.0 Hz and 5.1 Hz and pulse duration of 75 μs for 10 seconds, generating NVB-triggered electrical activities (Fig. 3) at before and after prostate removal. This signal is based on direct conduction of cavernous nerve excitation stimulating CC. The result electrical response was similar to spontaneous or MNSE CC-EMG because all of these share the same electric circuit [4], and it was generated continuously throughout the entire stimulation and sometimes after stimulation within a latency of 10 s (Fig. 3-B). Unilateral stimulation of each cavernous nerve resulted in bilateral triggered CC-EMG because this nerve conveys electrical activity through plexus passing the prostate and nerve endings are located intermingled near CC.
Neither postoperative incontinence nor impotence aggravated through sparing of cavernous nerve signals by IONM of CC-EMG. International index of erectile function-5 (IIEF-5) score of 24 improved as 21 at 6 months after operation. The total score of expanded prostate cancer index composite for clinical practice (EPIC-CP), a scale of quality of life for those after prostatectomy, was reported to be only 5 preoperatively, gradually improved as 5, 8, 10 at 1, 3, 6 months after operation, respectively.
Discussion
CC-EMG is the most direct neuro-electric response to instantly capture any injuries within the integrity from cavernous nerves to CC circuits correlated with erectile function [2]. Although other conventional intraoperative monitoring for ED used recording of pressure changes in cavernous bodies or subjective observation of tumescence [6–8], this case described how to successfully capture electric signals of CC- EMG during prostatectomy.
Cavernous nerve contains both sympathetic and parasympathetic nerve fibers which are travelling together [5]. Whether parasympathetic or sympathetic activation did not cause difference in CC-EMG generation because the same electric circuit is transmitting action potentials regardless of the origin of stimulation [3]. All three types of CC-EMG generated similar morphology and pattern of waveforms of a low-frequency, polyphasic signals (Fig. 2, Fig. 3). This had also been reported in a previous study about CC-EMG from healthy volunteers in an electromyography room [4]. In fact, the true electric-generating source of CC- EMG is not yet clearly known, either direct electric response from cavernous nerve excitation or impedance change of CC vasculature [3]. Intact cavernous nerves as well as healthy corpus cavernosal endothelial cells, adequate blood flow, and profound musculatures seem to be important to generate CC-EMG potentials.
Although simultaneously recorded from both surface and needle electrodes, needle electrodes showed less background noise. Still, noninvasive surface electrodes represented waveforms clear enough to interpret amplitude or frequency of all three kinds of CC-EMGs. Furthermore, more signals from ANS activity could be captured through surface electrodes than needle, because surface electrode covers larger areas of CC generating electric potentials. Regarding stimulation, an individual cavernous nerve mapping through stimulating the circumference of the prostate capsule requires at least 12 locations to stimulate for localization. This was too time-consuming to practically apply and NVB stimulation was chosen instead. The NVB was demonstrated to determine cavernous nerve integrity and erectile function through cadaver study [9]. Peripheral autonomic injuries could be suspected if amplitude and duration of CC-EMG signals diminish, whereas irregular shape and slow depolarization are more suggestive of CC smooth muscle degeneration or vascular causes [10]. However, there has been no normative data or any validated cut-off alarming criteria which is correlated with clinical prognosis to be used for cavernous nerve IONM yet.
Despite technical difficulties of ANS monitoring during IONM, CC-EMG can be a valuable method especially for those with pre-operatively intact potency to preserve erectile function and fertility. The NVB-triggered CC-EMG responses are useful because this feasible mapping of cavernous nerves generate same signals with spontaneous CC-EMG but take only 10 s. On the other hand, MNSE CC-EMG did not provide any additional information more than spontaneous or NVB-triggered EMG. Careful and thorough IONM of low sacral levels including CC-EMG monitoring during operation at pelvic area will provide further information and benefits to prevent unnecessary ED. Refinements of CC-EMG monitoring techniques and validation of how to interpret or quantify its signals should follow afterwards.
