Introduction
The purpose of intraoperative neurophysiologic monitoring (IONM) is to minimize neurological injury during surgery [1]. To achieve accurate and safe monitoring, a multimodal approach in IONM is recommended [1]. It has been regarded as a useful and effective method to avoid neurologic damage during surgery. However, routine IONM modalities, including motor evoked potentials (MEP), and somatosensory evoked potentials (SEP), only cover the sensory or motor system (spinothalamo-cortical or corticospinal tracts). Non-motor or sensory structures were not involved in these electrophysiologic surveillance systems. There were several reports about postoperative neurological or image change without IONM changes [2,3]. Most of these previous reports indicated that routine IONM did not detect lesions that involve the frontal lobes or caudate nucleus area during surgery [2–4]. Understanding this would be important for the development of IONM. Herein we report a case of monitoring failure without any neurophysiologic or hemodynamic change during surgery.
Case Report
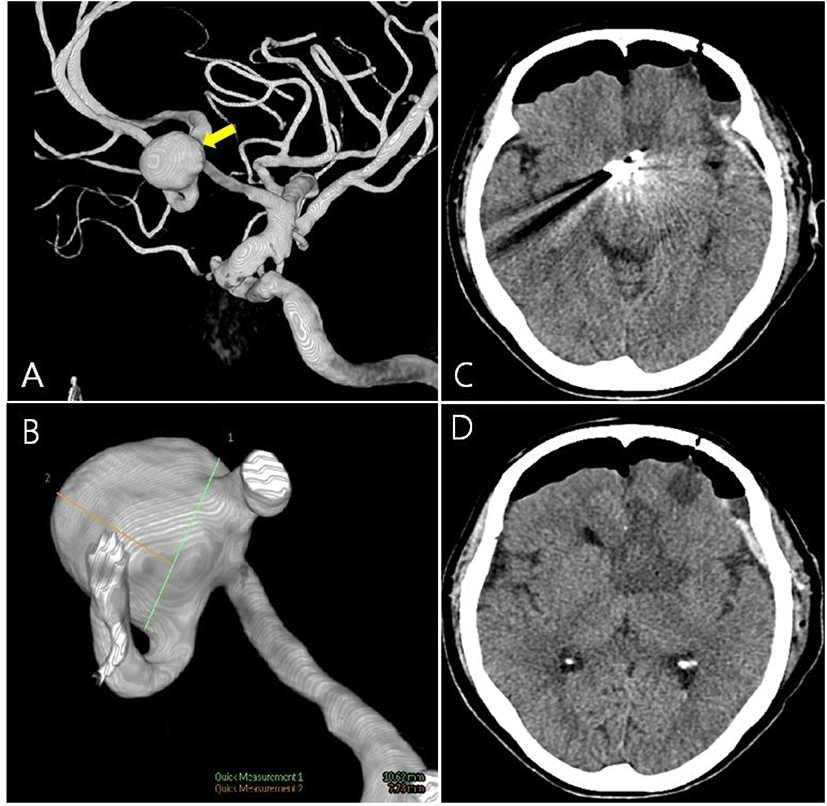
A 72-year-old female complained of severe headaches for over 1 year. Her symptom aggravated gradually. She had a history of hypertension on medication. On neurological examination revealed no abnormal findings. An unruptured aneurysm of the anterior communicating artery (Acom; 10.0 × 7.7 mm) was found on conventional angiography (Fig. 1-A, Fig. 1-B). We planned to perform aneurysmal clipping surgery.
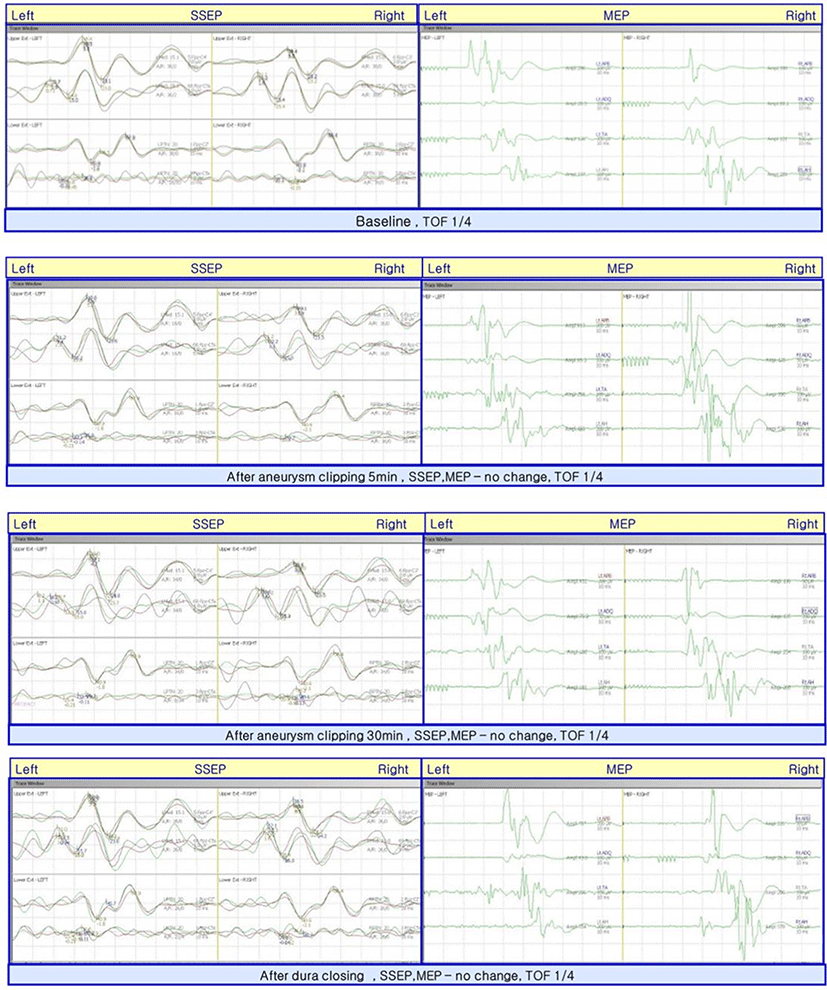
For monitoring her neurological function, SEP, MEP, and free-running EMG were used. For transcranial electrical stimulation, a pair of subdermal electrodes were placed at C3/C4 position. Transcranial electrical stimulation is performed five times in succession at 400 volts, 0.5 to 1.0 milliseconds each, with 2 milliseconds between each stimulation. Myogenic responses were recorded in bilateral abductor policis brevis, abductor digiti minimi, tibialis anterior, and abductor hallucis muscles. The sensory system were monitored by SEP. For stimulation of SEP, subdermal electrodes were positioned in the median nerve and the posterior tibial nerve on both wrists and ankles. Stimulation was delivered at 200 ms intervals at 20–30 mA. Recording electrodes were placed in Fz, Cz, C3, and C4. All electrodes were placed according to the international 10–20 system. General anesthesia was induced and maintained with propofol, vecuronium, and remifentanil. A neuromuscular blocker (vecuronium) was administered before endotracheal intubation. During IONM, the degree of neuromuscular blockade was monitored under train-of-four (TOF) monitoring.
At the point of clipping of the A-com aneurysm, we monitored MEP and SEP before and after the aneurysm clipping. There was no change in all modalities (Fig. 2).
After the surgery, the patient became in an abulic state. The patient showed spontaneous eye opening but did not respond to the oral command. Cranial nerve function tests were normal. Right after the surgery, motor functions showed a symmetric response to noxious stimuli and measured as medical research council (MRC) grade 5, but over time, the strength of right-side extremities decreased to about MRC grade 4+. Brain CT revealed low density in the frontal lobe (Left anterior cerebral artery [ACA] territory; Fig. 1-C, Fig. 1-D). Several months after the surgery, the patient’s abulic state was improved.
Discussion
We demonstrated a case of A-com aneurysm which resulted in ACA territory infarction without any IONM change during surgery. Motor and somatosensory functions were monitored through MEP and SEP several times before and after aneurysm clipping, but any significant changes were observed.
Until now, reports of postoperative abulia have been made in several studies [3–5]. Abulia is known as primarily associated with the anterior cingulate cortex and various subcortical connections such as a cingulo-opercular network [6]. In IONM, electrical stimulations and responses were delivered by large axon fibers like the corticospinal tract or posterior column tract. But anatomical structures which induce abulia are not located in IONM-detectable areas. In this case, the site of cerebral infarction was the prefrontal area of the left ACA territory (A2), which is distant from the main motor pathway. In postoperative brain CT, low densities were observed in the left caudate nucleus, anterior limb of the internal capsule (Fig. 1-C, Fig. 1-D). This area is usually supplied by a deep perforator such as the recurrent artery of Heubner. It arises near A-com to the A2 junction area. Clipping A-com would induce ischemia in these areas like our case. Due to the connective network of the caudate nucleus and prefrontal area, a lesion of the caudate nucleus would induce abulia or loss of motivation [6–8]. In addition, a corticospinal tract which carries major motor function, is in the posterior limb of the internal capsule, so motor deficit during surgery, in this case, might not be evident. The previously reported postoperative abulia cases were involved in the caudate nucleus or Acom lesions [4].
Other factors may also affect the ambiguity of IONM results. The use of neuromuscular blocking (NMB) agents could influence IONM during surgery. In previous research, the non-NMB use group showed higher accuracy than the partial use group [5]. In our case, TOF was 1/4 during the entire surgery. Both before and after aneurysm clipping showed the same TOF response (1/4), but the influence of the NMB agent could not be completely discounted. And the electrophysiologic aspect may also have an influence. High-intensity stimulation could stimulate deeper subcortical structures and induce bypass of the ischemic area [4]. Although we stimulate an appropriate intensity (400 V) in MEP monitoring, the possibility of deep structure penetration should be considered. And it is also necessary to consider and prevent other possibilities, such as silent ischemia or post-operative stroke [2,4].
Unfortunately, many efforts have been made on IONM, but the means to clearly monitor the areas, such as the prefrontal or caudate nucleus, which can cause abulia, are unclear. However, previous researchers suggested alternative intraoperative monitoring methodologies to overcome uncertainty in IONM. It has been reported that continuous electroencephalography could reveal changes impeding ischemia in an early phase [9]. Micro-doppler ultrasound would be another option for directly detecting flow loss after aneurysm clipping [10]. In conclusion, research on a new methodology for surveillance of the monitoring-vulnerable areas of the brain and research on appropriate monitoring strategies will be needed in the future.
