Introduction
Intraoperative neurophysiologic monitoring (IONM) is a comprehensive multimodal technique that includes somatosensory evoked potentials (SEPs), motor evoked potentials (MEPs), and electromyography (EMG) to preventive neural injury in spine surgery [1]. IONM enables the real-time monitoring of the functional integrity of nerves roots or spinal cord. Furthermore, these modalities are employed for mapping to identify neural structures, facilitating selective surgical approaches. There are existing evidences which support the efficacy of these modalities during the spinal surgery, including spinal cord tumor, spinal deformity, and degenerative diseases [2,3,4].
For intradural-extramedullary (IDEM) tumor, constituting two-thirds of primary spinal neoplasm, surgical resection is the most preferred treatment, providing spinal cord decompression [5]. Due to the potential risk of nerve damage during the surgical resection, IONM for IDEM tumor resection is performed to minimize neurologic deterioration [6]. However, the evidence supporting its efficacy specifically for IDEM tumors remains limited [5,7]. Nevertheless, considering the likelihood that an IDEM tumor may invade spinal nerves or the spinal cord, and the potential for damage to these structures during surgery, the routine implementation of IONM in IDEM tumor removal surgeries is a common practice across many medical centers.
In current study, we aim to report a case where the multimodal IONM was incorporated to map and identify the nerve roots from the sizable IDEM tumor, ultimately resulting in the absence of neurological sequelae. Particularly, this study emphasizes the crucial role of free-running and triggered electromyography (fEMG and tEMG) in determining the surgical strategy in IDEM tumor resection surgery.
Case Presentation
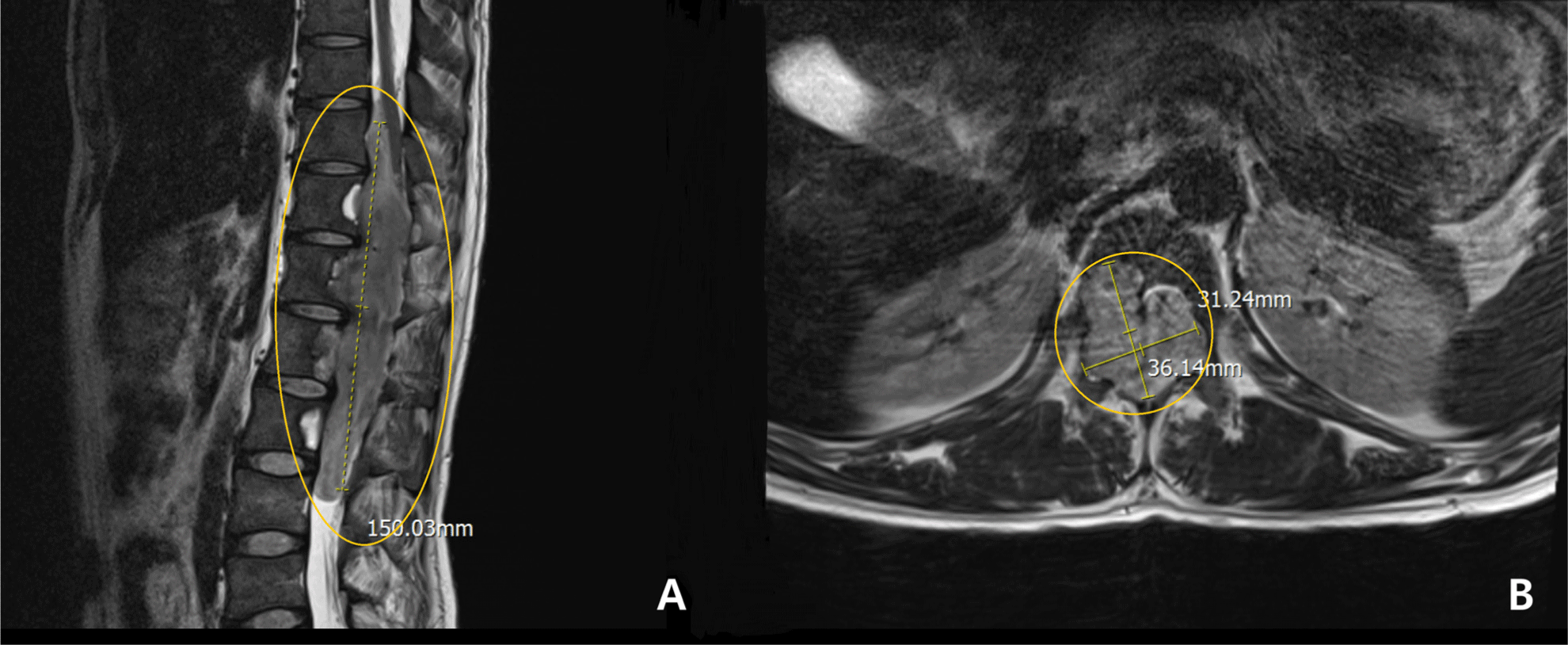
A 32-year-old male patient presented with progressive weakness in both lower extremities, resulting in gait disturbance, along with bowel and bladder dysfunction. Diagnostic magnetic resonance imaging revealed IDEM tumor at the T10-L3 level (Fig. 1). Motor scores for the bilateral 10 key muscles including elbow flexors (C5), wrist extensors (C6), elbow extensors (C7), finger flexors (C8), finger abductors (T1), hip flexors (L2), knee extensors (L3), ankle dorsiflexors (L4), long toe extensors (L5) and ankle plantar flexors (S1), were assessed by a clinician according to the Medical Research Council scale and the International Standards for Neurological Classification of Spinal Cord Injury. The motor scores were recorded as grade 2 for muscles corresponding to bilateral L4 and grade 1 for those corresponding to bilateral L5 and S1. The patient exhibited bilateral paresthesia and hypesthesia below the L1 dermatome, with decreased bulbocavernous reflex (BCR) and reduced deep tendon reflex in the knees. To prevent further neurological deterioration and possibly regain function, a posterior laminectomy from T9 through L3, along with tumor removal, was planned with the use of IONM.
The IONM involved the use of transcranial electrical stimulation MEPs and fEMG of the bilateral psoas, tibialis anterior (TA), and abductor hallucis (AH) muscles. Bilateral abductor pollicis brevis muscles were also monitored, serving as reference data. Paired recording needle electrodes were inserted into these relevant muscles, with the psoas muscles being accessed from the inguinal area. A single train stimulation, consisting of 6 pulses (pulse width of 0.05 ms; interpulse interval of 3.0 ms), was administered at an intensity of 300–350 V using the interhemispheric montage between C2 and C1. Simultaneously, SEPs from the bilateral tibial nerves were recorded, employing a stimulation intensity of 16–20 mA (pulse width of 0.3 ms; frequency of 4.7 Hz) applied at the posterior side of the bilateral lateral malleolus, with recordings taken from the Cz-Fz montage.
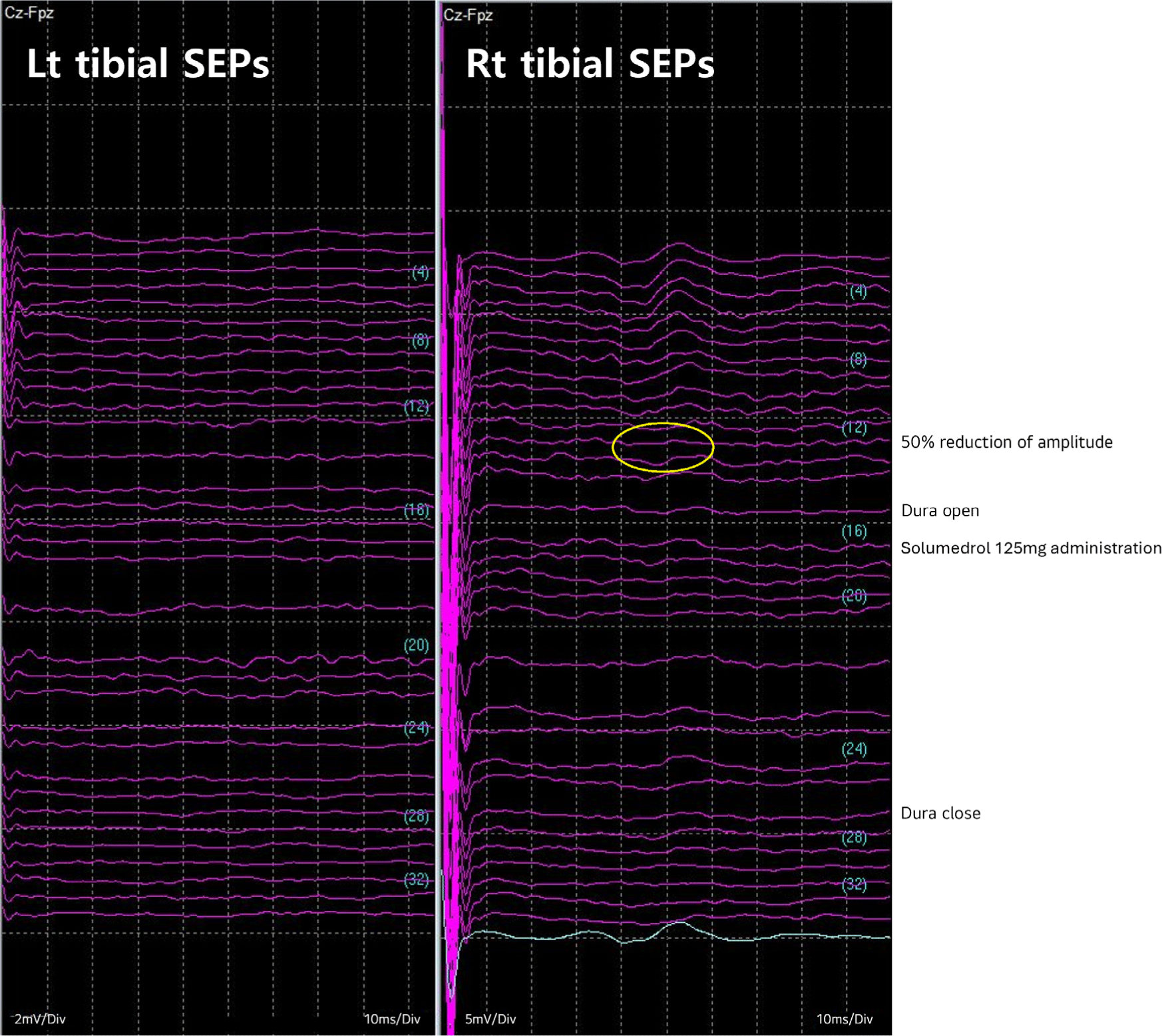
Total intravenous anesthesia was employed, along with a one-time 50 mg intravenous dose of rocuronium for intubation. Throughout the surgery, the patient remained in a prone position. Baseline waves of bilateral tibial SEPs were acquired immediately after intubation. However, the SEPs of the left tibial nerve displayed no response from the baseline. The baselines for MEPs were obtained after the effect of rocuronium faded out. During the entire surgery procedure, no specific abnormalities were observed in MEP monitoring. However, during the laminectomy of right T9-L3, SEPs of right tibial nerve decreased by more than 50% compared to baseline (Fig. 2), and it was promptly reported to the surgeons and anesthesiologist. Despite the administration of a 125 mg dose of methylprednisolone (Solu-Medrol®, Pfizer, New York, NY, USA), no recovery was noted until the end of the monitoring.
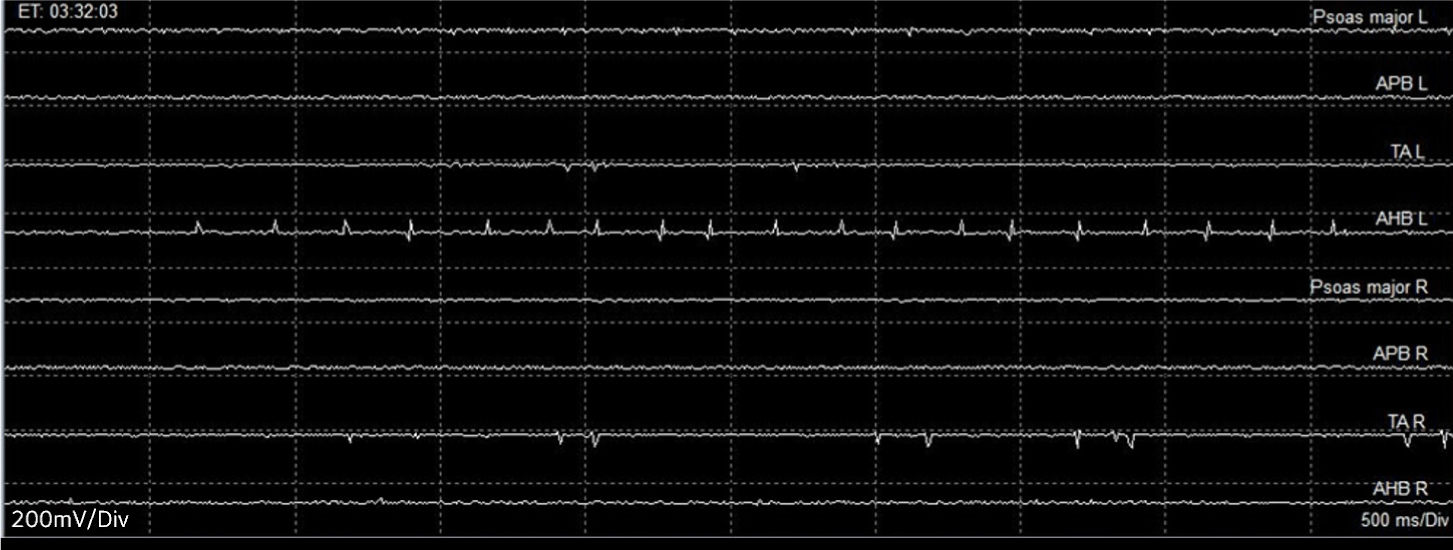
During the tumor removal procedure, B-train with spikes activity was frequently detected in left TA and AH muscles on the fEMG monitoring while the tumor was being retracted. This prompted an immediate notification to the surgeon (Fig. 3), leading to a modification of the surgical plan from the en-bloc removal to the segmental tumor removal. The B-trains disappeared from monitoring upon the closure of dura.
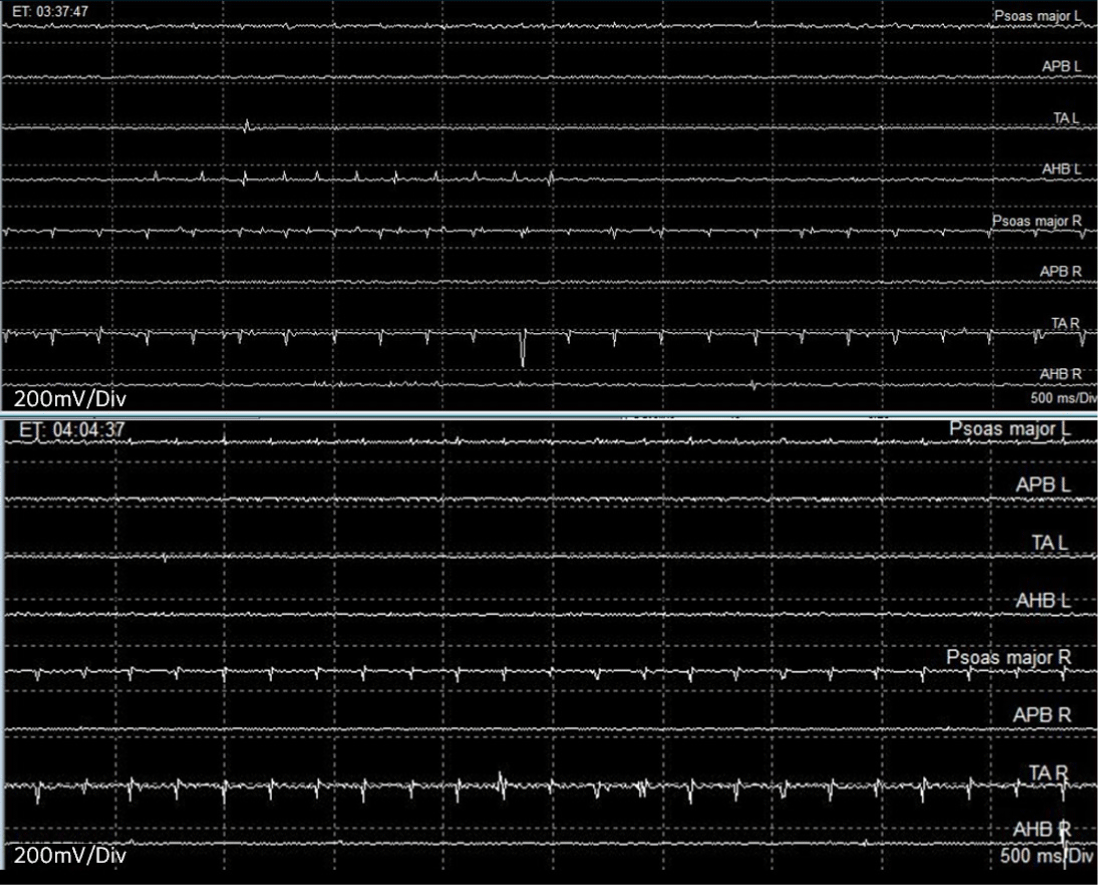
Due to the extensive invasion of the spinal levels by the large tumor (Fig. 1) and the potential entanglement of the tumor with the nerve roots, which posed a high risk of sacrifice, tEMG was additionally planned to discern between the nerve roots and soft tissue. The surgeon used a disposable ball tip monopolar stimulator (Disposable Ball Tip Direct Nerve Stimulator Probe, Cadewll Industries, Kennewick, WA, USA) to explore the potential presence of nerve roots within the intermingled soft tissue. The stimulating intensity ranged from 0.2 to 2.0 mA, with a pulse duration of 0.5 ms and an interpulse interval of 3 ms. Recording was conducted in the same muscles used for fEMG monitoring. During tEMG-based mapping, compound muscle action potentials (CMAPs) were detected in bilateral psoas, TA or AH muscles at several tumor-related sites, and these responses were promptly notified to the surgeon (Fig. 4).
Throughout the surgery, there was no significant deterioration in monitored vital signs, including blood pressure, respiration rate, oxygen saturation and pulse rates.
Two days post-surgery, the neurologic state of the patients was assessed, revealing no further deterioration in motor and sensory function compared to the preoperative status. The patient was then transferred to the Department of Rehabilitation Medicine for functional training and education. Two weeks post-surgery, the patient’s motor score for right S1 and bilateral L5 key muscles were improved to grade 3, with no change in sensory function.
Discussion
This case highlights the outcomes of IDEM tumor surgery with the incorporation of IONM, with a specific emphasis on the role played by fEMG and tEMG, as they can significantly impact the surgical plan. It revealed the efficacy of these modalities, additional to the previous studies, by warning the surgeons of the root involvement leading to the modification of surgery, thus minimizing the postoperative complication.
In this case, the IDEM tumor was located extensively between the T9-L3 levels, and the patient exhibited symptoms of cauda equina syndrome including bowel and bladder dysfunctions, along with decrease BCR and deep tendon reflex of the knees. Postoperative neurological deficits are rarely associated with IDEM tumors of the cauda equina [6,8]. This has given rise to a debate regarding the necessity of IONM in IDEM tumor operations. However, despite the benign nature of IDEM tumor, the risk of recurrence is heightened if total removal is not achieved. Therefore, neuromonitoring is crucial for achieving a total removal of the tumor while efficiently avoiding a potential neural injuries [9]. In a previous study, the imperative role of IONM, with a specific emphasis on the role of tEMG, has been asserted for cauda equina tumor surgery [6]. The tEMG is used to identify the nerve roots, enabling the surgeon to make informed decisions about whether to leave a residual tumor to preserve the root or sacrifice the root for complete removal, thereby facilitating appropriate modifications to the surgical plan.
Unlike direct stimulation in tEMG, fEMG records the CMAP responses that are spontaneously elicited from nerve irritation from various surgical procedures. While there is no firm consensus on the alarm criteria for fEMG, train activities are often studied in relation to adverse neurological outcomes [10]. There are three types of fEMG activity, including the A-, B- and C-train. In this case, the B-train was captured during the retraction of the IDEM tumor mass. The B-train has been defined as a regular or irregular sequence of single components with maximum intervals of 500 ms, and it can last for several minutes or even hours [11]. Previous studies have indicated that B- or C-train activities were not correlated with postoperative paresis, whereas the A train showed a significant correlation with postoperative deterioration [11]. On the other hand, other study reported that both A- and B-train activities could serve as indicators for adverse neurosurgical outcomes [11]. Given that train activities can potentially serve as precursors to electrophysiologic deterioration, it is crucial to maintain close communication with the surgeons to reduce or minimize these activities, thereby helping to prevent postoperative neurological deterioration. In this case, no abnormalities were observed in MEP monitoring, which may be attributed to the result of close communication aimed at protecting the motor tract by modifying the surgical procedure based on information provided by fEMG and tEMG.
However, during the laminectomy, the amplitude of SEPs for the right tibial nerve decreased by more than 50% compared to baseline and did not show any recovery until the end of the surgery, even with the administration of methylprednisolone. Neither intraoperative MEP deterioration nor neurological paresis was observed in this case report, consistent with the results observed in previous study. In most of the prior cases, intraoperative SEP decay was observed upon opening the dura, and restoration occurred after closure of the dura during IDEM surgery [5]. In this case, the SEPs deteriorated and did not recover during the surgery. This may be attributed to spinal cord manipulation during the tumor resection, particularly in cases involving adhesions between tumor and nerve roots. Nevertheless, the fact that the patient did not exhibit further sensory deterioration could be attributed to either a specificity issue of the SEP monitoring or the possibility that any sensory abnormalities, if they occurred, might have improved within two days after the surgery.
This study has several limitations. Firstly, two weeks post-surgery, the patient demonstrated more improved muscle strength in lower extremities than that of immediate postoperative status. It is challenging to determine whether this improvement can be solely attributed to the effective decompression achieved through gross total tumor removal using multimodal IONM or if it could be a combined effect of postoperative rehabilitation, which might facilitate neural plasticity. Secondly, the patient still engaged in self-catheterization following surgery due to persistent voiding difficulties. A notable limitation lies in the absence of preoperative BCR studies and intraoperative BCR monitoring, as well as the absence of EMG monitoring from the BC muscles or external anal sphincters to assess the neurologic functions of the bladder and bowel.
In conclusion, this report highlights a case where there was no postoperative neurological deterioration after IDEM tumor surgery with multimodal IONM, utilizing a combination of MEPs, SEPs, fEMG and tEMG. Particularly, this case report emphasizes the role of tEMG and fEMG in rapid decision-making for modifying sequential surgical procedures, as well as the use of evoked potentials to precisely avoid the neural damage in IDEM tumor that invade multiple lumbar nerve roots.
