Introduction
Intraoperative neurophysiological monitoring (IONM) for spinal cord tumor surgery has become increasingly common prevent the postoperative neurological complications. However, while motor and somatosensory evoked potential (SEPs) monitoring are fundamental modalities for IONM and are included in clincial practice guidelines with level I clinical evidence, intraoperative electromyography (EMG) is often underestimated and less often monitored. Here, we report a clinical case of IONM during spinal cord tumor surgery in which intraoperative EMG provided a crucial role.
Case report
A 79-year-old man presented to a tertiary hospital with radiating pain and progressive weakness in bilateral lower extremities over a period of six months. The motor scores for 10 key muscles in bilateral lower extremities were graded as 4 according to the Medical Research Council scale and the International Standards for Neurological Classification of Spinal Cord Injury. His sensory function was normal, and bladder and bowel functions were preserved. Magnetic resonance imaging revealed a cystic spinal cord tumor at the T12-L1 spinal level, and tumor removal surgery was planned with IONM (Fig. 1-A). The IONM was designed to include motor evoked potentials (MEPs), SEPs, free running EMG (fEMG), and triggered EMG (tEMG), but not D-wave monitoring, as the level was below T12. The MEPs and fEMG monitored bilateral tibialis anterior and abductor halluces muscles. Bilateral abductor pollicis brevis muscle werer also monitored for reference data. A single train stimulation, composed of 6 pulses (0.05 ms pulse width, 3.0 ms interpulse interval), was delivered at 300–350 V using an interhemispheric montage between C2 and C1. Concurrently, SEPs were recorded from the bilateral tibial nerves with a stimulation intensity of 17 mA (0.3 ms pulse width, 4.7 Hz frequency) applied to the posterior side of the bilateral lateral malleolus, with recordings taken from the Cz-Fz montage. Throughout the surgery, the patient was maintained in a prone position. Baseline waves of bilateral tibial SEPs were obtained immediately after intubation, while baselines for MEPs were acquired after the effect of rocuronium had dissipated. During surgery, MEPs and SEPs showed no significant deterioration (Fig. 2-A and 2-B).
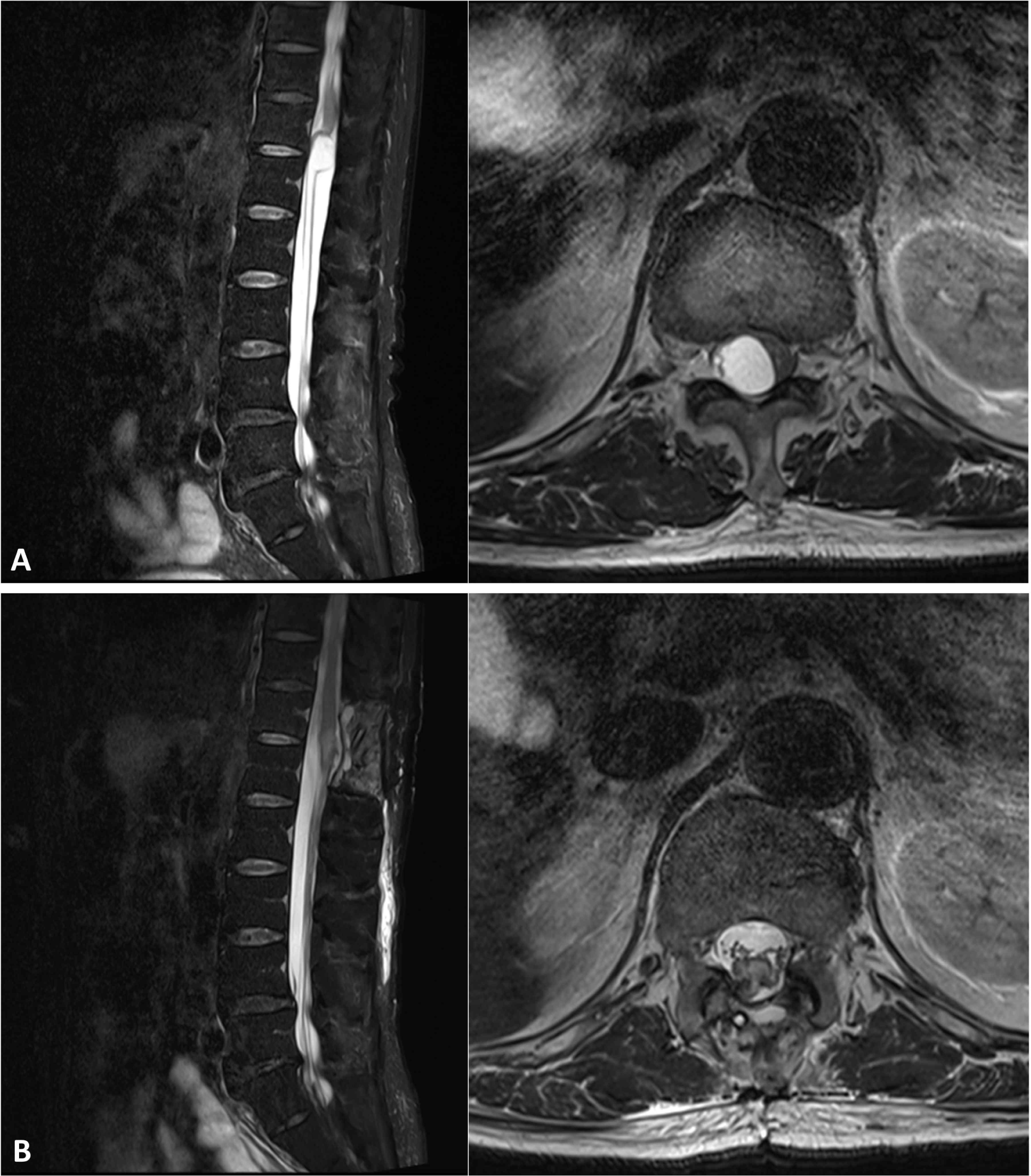
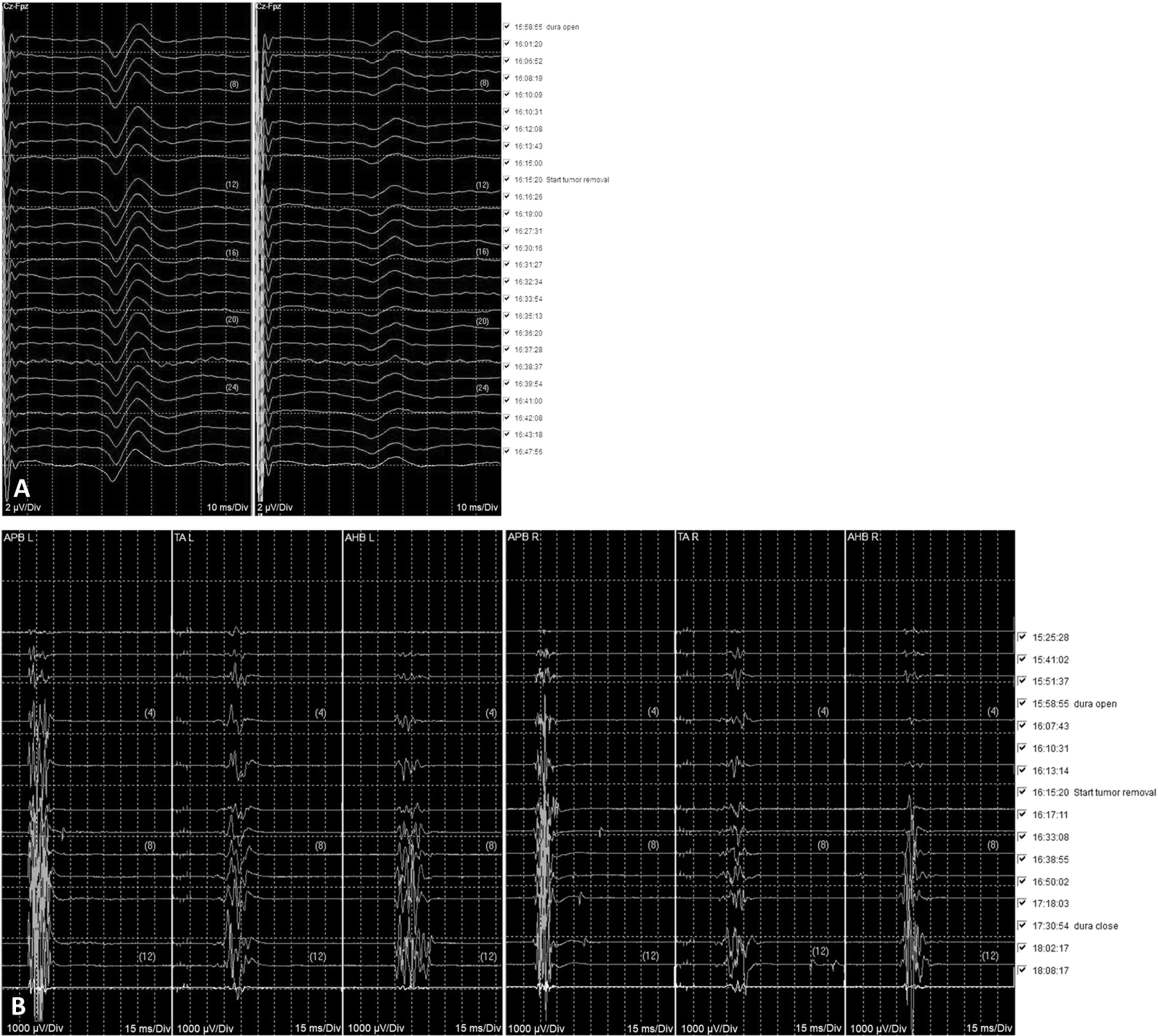
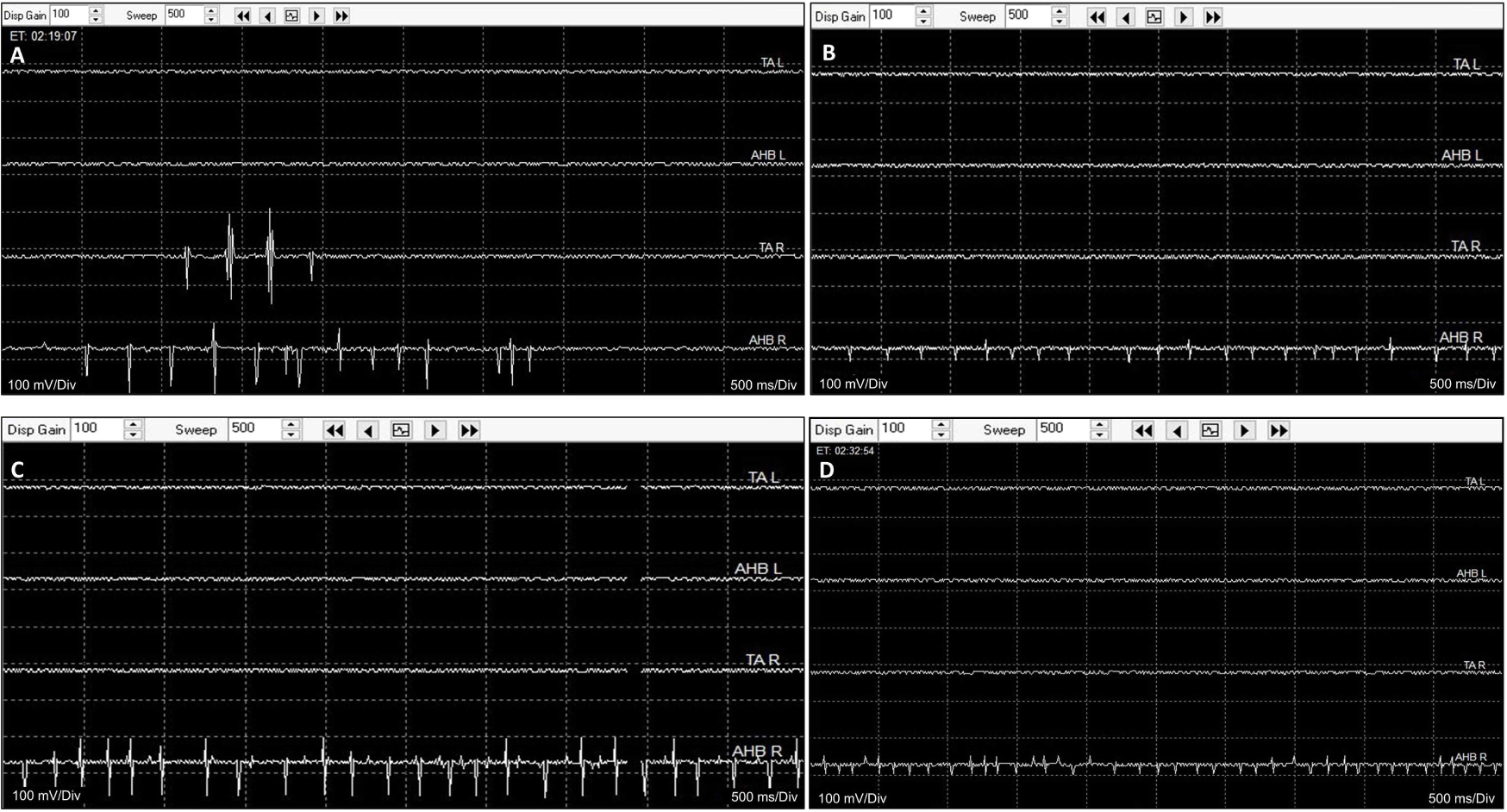
The surgeon ruptured the tumor cyst, and carefully made internal decompression of the tumor using cavitronic ultrasonic surgical aspirator. During tumor retraction, fEMG showed B-train activities were observed in right tibialis anterior and abductor hallucis muscle, of which promptly notified to surgeon (Fig. 3-A and 3-B). The IONM professional suggested to the surgeon the use of tEMG for identifying spinal nerves, along with the monopolar stimulator. The stimulator was already configured to insert a subdermal needle reference electrode into the paraspinal muscles near the surgical site but outside the surgical field. The surgeon placed a disposable ball-tip stimulating probe in areas where he suspected the presence of spinal nerves. Stimulation was given with 0.2–2.0 mA intencity with 0.3 ms pulse width and 4.7 Hz frequency, simply using the above preset SEP stimulating setting in the monitoring machine. During tEMG exploring, compound muscle action potentials were detected in right abductor hallucis muscle during the tumor removal at conus medullaris lesion (Fig. 3-C and 3-D). The surgeon meticulously excised the tumor where triggered potentials were absent. However, in the soft tissue heavily adhered to the main tumor lesion, consistent triggered signals hindered safe dissection from the spinal nerves. The intraoperative frozen biopsy pathology revealed an ancient schwannoma. Consequently, the surgery, initially planned for en-bloc resection, was halted, leaving some lesions in the conus medullaris to preserve neurological function (Fig. 1-B). All channels of MEPs showed increased amplitudes compared to the baslines after tumor removal, persisting until the end of the surgery, and the SEPs showed no significant deteioration during the entire surgical procedure. The patient experienced no motor deterioration post-surgery but did report transient numbness lasting for several days in the right L5 and S1 sensory dermatome.
Discussion
This case study demonstrates how real-time fEMG and tEMG monitoring enabled the surgeon to modify the surgical plan and successfully complete the operation without any motor deficits. During surgery, neural insults can provoke signals in the innervating muscle. Patterns of fEMG signals, such as spikes, bursts, neurotonics, myokimic discharges and train activities (A-train, B-train, and C-train), are considered hazardous [1]. Myokimic discharges and neurotonic discharges are also described as train activities [1,2]. Although the term “neurotonic discharge” is still in use during IONM, “myokymic discharge” is mostly substituted for “train activity” despite the unclear pathophysiological mechanism of these patterns [2,3]. In addition, there are no quantitative warning criteria for the amplitude, duration, or frequency of abnormal fEMG signals in relation to postoperative neurological deterioration. Even with further data accumulation, it would be difficult to establish firm criteria because, unlike SEPs or MEPs, fEMG cannot obtain a baseline EMG activity due to its normal silent state. Skinner et al. investigated the reliability of fEMG during intramedullary spinal cord tumor resection and proposed the following warning criteria: 1) irregular aperiodic EMG bursts were repeatedly elicited by similar surgical maneuvers within the tumor bed; 2) prolonged (> 3 sec), focal, semirhythmical tonic discharges occurred with or without an obvious surgical event; 3) an active EMG signal in one or more limbs became acutely silent [4]. Despite the study’s significant limitation of a small sample size of only 14 cases, the sensitivity and specificity of fEMG according to these criteria were 87.5% and 83.3%, respectively, while those of MEPs were 75.0% and 100%, respectively. This suggests that fEMG is better for detecting neurological insults, whereas the prognostic value is higher for MEPs. Thus, fEMG is a complementary modality for motor tract monitoring and should be performed alongside MEPs. Even, it can share the recording electrodes for MEPs, if the target muscles are the same. This fEMG is also being studied to enhance IONM sensitivity and negative predictive value during metastatic spine tumor surgeries when combined with MEP and SEP monitoring [5]. If significant fEMG is detected, it should be promptly reported to the surgeons so they can check the surgical field, and the MEPs and SEPs should be promptly reviewed to see if there are any accompanying deteriorations in EPs, which have a consensus warning criteria.
IONM professionals should distinguish hazardous fEMG signals from the numerous artifacts that arise from electrocautery, cold saline irrigation, light anesthesia, movement, SEP stimulation, and other sources. Especially, the artifact caused by continuous cold saline irrigation is similar to C-train. In addition, the SEP stimulation artifact can be mistaken for a burst train consisting of consecutive spikes. Indeed, the spikes, bursts, or B-train may overlap with SEP stimulation artifacts, which can hinder the proper detection of these abnormal fEMG activities. This underscores the importance of continuous monitoring by a skilled examiner.
During spinal cord tumor surgery involving the conus medullaris or spinal nerves, the use of tEMG should be considered an essential medical practice. In cases where the nerve root and tumor mass are entangled and indistinguishable, even preoperative magnetic resonance image currently cannot precisely determine the surgical resection area, and there is no imaging tool available to view the internal structures of the tumor during surgery. So far, the only method for mapping the spinal nerves is through electrophysiological techniques using tEMG. In this case, tEMG played a crucial role in guiding the surgical strategy during the surgery, as the surgeon changed the plan from en-bloc resection to segmental resection, which resulted in a favorable motor prognosis. A recent case report also supports this context, highlighting the use of fEMG and tEMG during tumor resection involving the conus medullaris and spinal nerve roots in a tumor with a complex nerve conglomeration [6]. In addition, a study reported the sensitivity of tEMG is superior to MEP in predicting postoperative neurologic deficit during cauda equina tumor surgery [7]. The tEMG also helps to identify nerve roots with lipomas of filum terminale [8]. However, tEMG does not always detect nerve roots that are intermingled within the tumor; when such detection fails, there have been reports of worsened postoperative motor outcomes due to false-negative results [3].
There are some limitations in this case report. Firstly, the patient could not undergo preoperative electrodiagnosis, which provides specific electrophysiological information for designing IONM modalities. Consequently, the IONM design relied solely on the patient’s symptoms. Additionally, the number of muscles in the bilateral lower extremities monitored during surgery was limited. Although no weakness occurred postoperatively, this restriction could potentially overlook neural injuries of the upper lumbar spinal nerves, such as L2, L3, or L4.
This study highlights the importance of utilizing fEMG and tEMG in ensuring the safety of spinal cord tumor surgeries involving the spinal nerves. However, due to the lack of quantitative criteria for fEMG, the emphasis on multimodal IONM should still be maintained.
