The surgical outcomes of patients with cerebral aneurysm may be severely impaired by ischemic complications resulting from inadvertent vascular occlusion or compromise [1–4]. Incorrect placement of the clip may cause incomplete exclusion of the aneurysm or compromise blood flow in the adjacent arteries [1,3,4]. Herein, we report a case in which blood flow insufficiency after the release of the brain retractor was detected through intraoperative neurophysiological monitoring (IONM) and ischemia was successfully avoided.
Case report
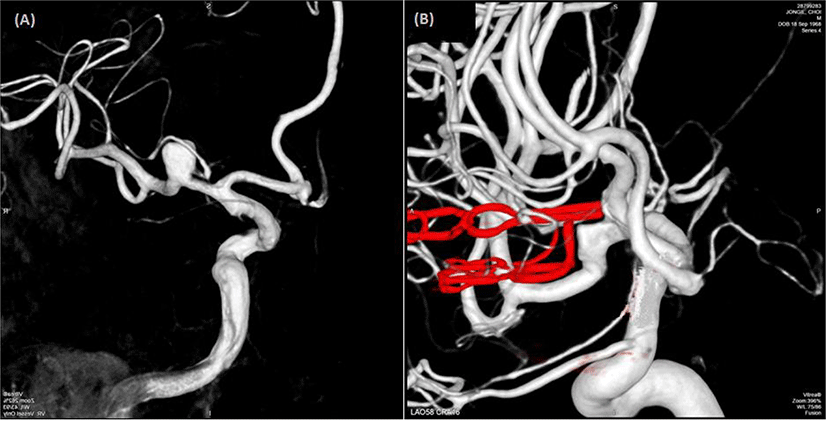
A 49-year-old man visited our hospital because of an asymptomatic unruptured intracranial aneurysm identified using magnetic resonance angiography. Cerebral catheter angiography revealed a saccular aneurysm at the M1 segment of the right middle cerebral artery (Fig. 1-A). Surgical clipping was performed with IONM.
Total intravenous anesthesia with propofol (3.5–4.0 μg/mL) and remifentanil (1.0–3.5 μg/mL) was used. Neuromuscular blockade was applied immediately before intubation (0.5–0.9 mg/kg rocuronium) without a maintenance dose.
Somatosensory evoked potentials (SSEPs) and transcranial motor evoked potentials (tcMEPs) were monitored during surgery. To obtain SSEPs, we administered square-wave electrical pulses for 0.3 ms at 15 mA for the median nerve and 20 mA for the tibial nerve at a frequency of 2.31 Hz. Recording electrodes were placed at C3’, C4’, Cz’, fifth cervical spine, Erb’s point, and popliteal fossa. In addition, a reference electrode was placed at Fpz. Low- and high-band pass filters were set at 30 and 1,000 Hz, respectively.
TcMEPs were evoked using an electrical stimulator (Xltek Protektor 32 IOM system; Natus Medical Inc, Ontario, Canada). A train of five square-wave stimuli was delivered with an individual pulse duration of 0.05 ms, an inter-stimulus interval of 1–2 ms, an intensity of 250–500 V, a time base of 100 ms, and a 10–1,000-Hz filter. C1 anode and C2 cathode pairs were used for stimulation of the left hemisphere, and the reverse arrangement was used for the right hemisphere. MEPs were recorded using subdermal needle electrodes placed in the abductor pollicis brevis (APB), abductor digiti minimi, tibialis anterior, and abductor halluces muscles.
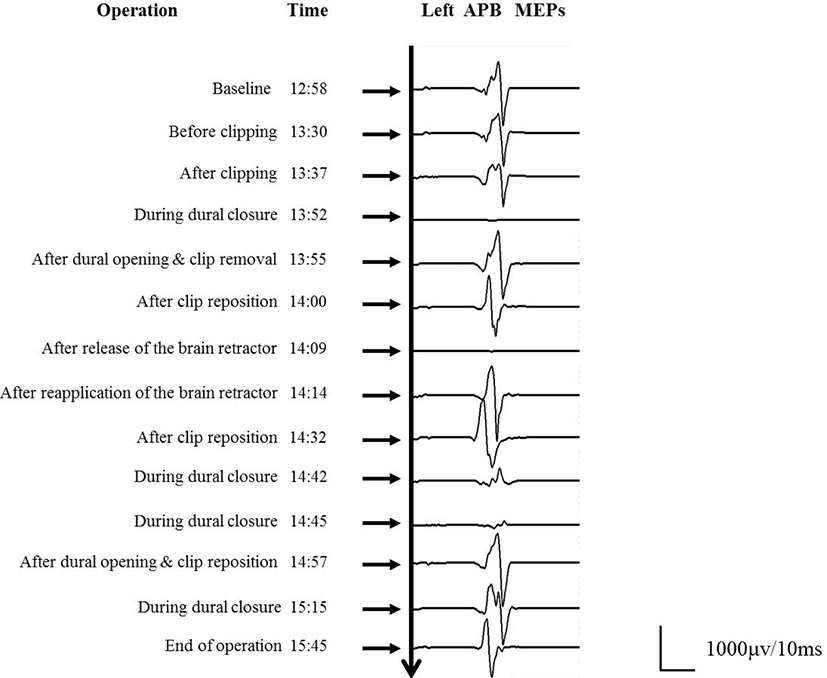
A standard frontotemporal craniotomy was performed. After exposure of the aneurysm, its neck was clipped using a downward curved clip. Subsequently, two stacked clips and a fenestrated booster clip to reinforce the closure were applied to the aneurysmal dome and distal neck, respectively. Indocyanine green (ICG) angiography showed that all of the vessels were patent and non-compressed, and the amplitude of SSEPs and MEPs remained at the control level. Approximately 15 min after clipping, during dural closure, isolated loss of the left APB MEP was observed. The surgeon reopened the dura and all clips other than the curved clip applied to the aneurysmal neck were rapidly removed, resulting in the gradual restoration of the left APB MEP. Two stacked clips were reapplied to the aneurysmal dome. ICG angiography confirmed blood flow in the adjacent arteries. Immediately after release of the brain retractor, the left APB MEP amplitude was lost again. The clips were suspected to have directly compromised blood flow by repositioning the frontal lobe and kinked the adjacent arteries after release of the brain retractor, and clip readjustment was needed. To minimize the likelihood of clip rotation, we clipped the aneurysmal neck using a curved fenestrated clip. A straight fenestrated clip and curved mini-clip were applied to the dome of the aneurysm. Furthermore, a large amount of oxidized cellulose (Surgicel®) and fibrin sealant (Tisseel®) was inserted between the frontal base and the clips. SSEPs and MEPs were maintained until the end of surgical closure. Intraoperative findings and MEP changes are shown in Fig. 2.
Postoperative cerebral catheter angiography revealed complete obliteration of the aneurysm and no compression of the adjacent arteries (Fig. 1-B) and a computed tomography scan (performed on Postoperative Day 5) revealed no new low-density areas. The patient was discharged 1 week after surgery without any neurologic deficits.
Discussion
Cerebral aneurysms often occur in locations surrounded by adjacent neurovascular structures, such as the optic nerve, the oculomotor nerve, and the cerebral arteries and their branches. Therefore, surgical outcomes may be severely impaired by ischemic complications resulting from inadvertent vascular occlusion or compromise [1,3,4]. The possible mechanisms of blood flow insufficiency during or immediately after aneurysm clipping surgery include clipping of the nearby parent or perforating vessels, thromboembolism, vasospasm, and clip rotation caused by postoperative edema in the neighboring structures [3,4]. Studies using intraoperative modalities to assess vessel patency have demonstrated the incidence of unexpected vessel compromise to be in the range of 5%–30%, and more than 50% of these patients experience stroke [5]. This complication is reversible in intraoperative settings, and should be avoided to optimize patient outcomes [2,5].
To clip an aneurysm successfully without injuring the surrounding cerebral structures requires appropriate planning and delicate operative techniques. Although the neurosurgeon may judge that clip placement has been performed adequately, clip rotation might occur and cause strangulation of a branching artery near the aneurysm after release of brain retraction [1,3,4,6,7]. Clip rotation may occur in the period between clipping and the end of general anesthesia. Several intraoperative monitoring techniques, such as micro-Doppler ultrasound and ICG angiography, have been developed to reduce ischemic complications after aneurysm clipping[1,2]. However, no method exists to verify distal blood flow after removal of the retractor and dural closure. In our case, blood flow through the arteries, which had been verified on ICG angiography, might have been compromised after the release of brain retraction. IONM could overcome this pitfall.
We reported a case with delayed loss of MEP during cerebral aneurysm clipping surgery, in which aneurysm clip rotation after release of brain retraction might have disrupted blood flow in the branching arteries near the aneurysm. We emphasize that surgeons and IONM personnel should not only exercise caution in the clip placement itself, but also be aware of the necessity of continuing IONM until the end of surgery, because major changes may occur even during the non-key portions of surgery.







