Cranial nerves can be damaged during a variety of surgical procedures. The function of the cranial nerves can be monitored during surgery with electromyography (EMG), motor evoked potentials (MEP), and brainstem auditory evoked potentials (BAEP). Free running EMG provides real-time feedback whenever a motor nerve is activated or irritated [1]. Neurotonic EMG activity lasting longer than 10 seconds has been associated with postoperative deficits; however, absence of abnormal spontaneous EMG activity is not necessarily indicative of intact nerve function [2]. Neurotonic EMG may be absent following serious nerve injury, including sharp dissection. Here we report a case of incomplete facial nerve palsy after schwannoma removal with neurotonic discharge; it was sustained for approximately 2 minutes and disappeared suddenly.
Case report
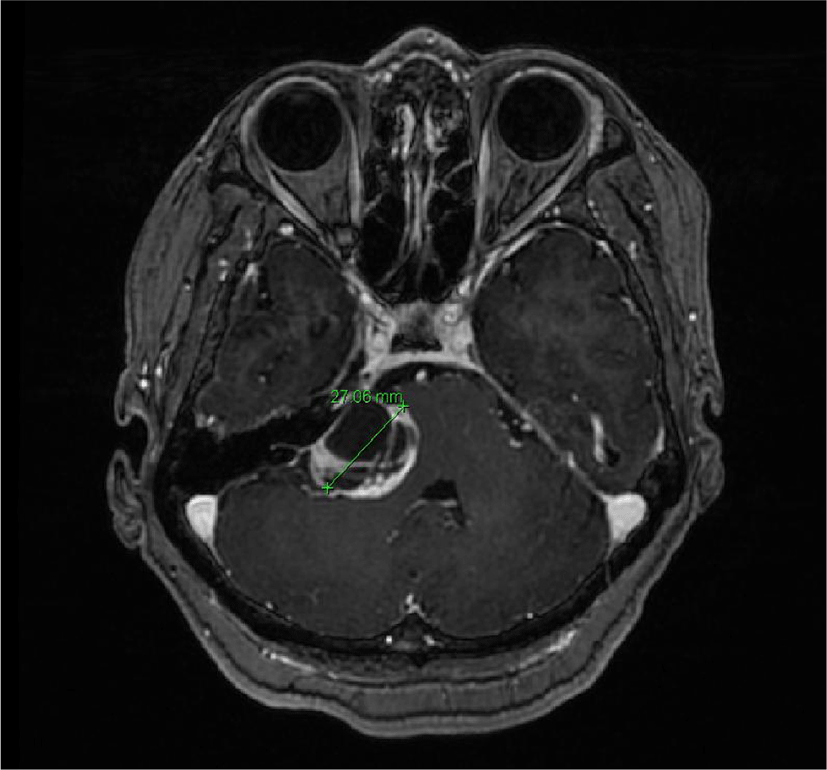
We report the case of a 63-year-old woman who had an acoustic schwannoma. She presented with tinnitus and hearing loss for 1-2 years and already had right facial palsy at House Brackmann (HB) grade II [3]. Her brain magnetic resonance image showed an approximately 3 cm cystic and solid mass in the right cerebellopontine angle with extension to the right internal acoustic canal, suggesting acoustic schwannoma compressing the pons and right middle cerebellar peduncle (Fig. 1). She was referred to us by evaluation in an electrodiagnostic study before surgery with a degeneration ratio of 50.0%.
MEP were obtained during the whole surgical process. Baseline MEP were obtained from the beginning of surgery. Muscle MEPs recorded from the both abductor pollicis brevis and deltoid muscles [4]. Somatosensory evoked potentials (SEP) were monitored sequentially during entire surgical process through bilateral median nerves. Bilateral median SEP were obtained and bilateral N20 latencies were checked as baseline. We stimulated the right and left median nerves at the wrist and recorded on left (C3’) and right (C4’) respectively [5]. BAEP were obtained during whole surgical process with baseline BAEP obtained at the beginning of surgery. We stimulated the left and right ears to obtain V waves [5]. Free running EMG were obtained during surgical process. The needle inserted into the bilateral frontalis, orbicularis oculi, orbicularis oris and mentalis muscles [5].
We monitored MEP, SEP, BAEP, and free running EMG. The MEP and SEP showed acceptable amplitudes during monitoring process; however, the BAEP showed no response from the beginning bilaterally.
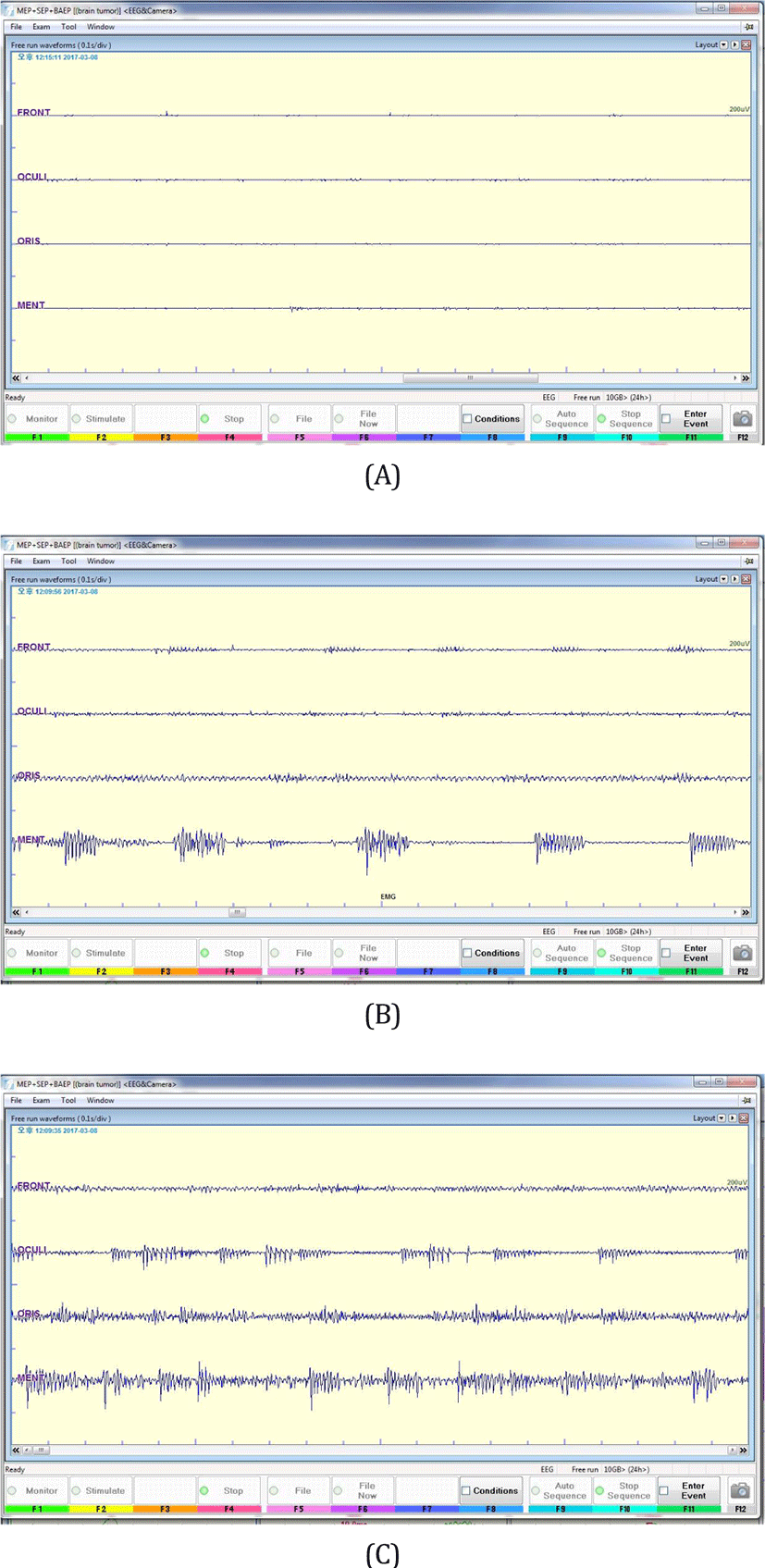
During tumor removal, free running EMG showed no abnormal discharge at first (Fig. 2-A). Suddenly we found an abrupt increase in activity in the left orbicularis oculi, orbicularis oris and mentalis muscles (Fig. 2-B, Fig. 2-C). The activity showed high frequency bursts of motor unit potential and the sound was similar to end plate spikes or myotonia. However, as the facial nerve was severely adhered to the schwannoma, the surgeon decided to continue removing the tumor. Neurotonic discharge was sustained for approximately 2 minutes and disappeared suddenly.
After surgery, she presented with worsened facial paralysis (HB grade IV). We performed EMG before and after surgery. Facial nerve trunks were stimulated by supramaximal stimuli at the stylomastoid foramens and compound muscle action potentials were measured through recording electrodes placed on the ala nasi [6]. which showed that the degeneration ratio increased from 50.0% to 85.3%.
Discussion
Intraoperative monitoring helps to detect nerve injury during surgical procedures and predict nerve function after the operation. Free running EMG provides immediate feedback and gives prompt information to the surgeon about nerve irritation or injury. This information can aid the surgeon in the assessment of neural structures. As false positive and negative errors can occur with monitoring, the surgeon and monitoring team should choose whether or not the surgical procedure should continue [4,5].
Burst potentials can be observed when the surgical instrument contacts the nerve and decreases with repeated nerve contact. There are other potentials such as a “train” potential, which is caused by mechanical injury or thermal changes. With increasing nerve injury, a greater intensity and longer duration of nerve potential becomes evident. Therefore, the surgeon can respond and can determine whether the surgery will continue [3].
In this case, the neurotonic activity could have been caused by nerve irritation or injury. However, the abrupt disappearance is likely explained by the loss of stimulation or complete nerve injury. If the disappearance of potential was due to loss of stimulation, the facial palsy of the patient would not worsen compared with her preoperative status. Therefore, the presence of facial palsy suggests the possibility of nerve injury.
Free running EMG provides real-time feedback to indicate if a motor nerve is activated or irritated. Neurotonic EMG activity lasting longer than 10 seconds has been associated with postoperative deficits. However, absence of abnormal spontaneous EMG activity is not necessarily indicative of intact nerve function. Neurotonic EMG may be absent following serious nerve injury, including sharp dissection. Therefore, it is difficult to determine whether the disappearance of neurotonic discharge is indicative of nerve irritation or severe injury to a peripheral nerve.
Also, one of the limitation of this study is the absence of corticobulbar MEP. Because the preservation of corcicobulbar MEPs for cranial nerves at the end of the surgery is a good prognostic factor [1].
In conclusion, surgeons and clinicians should be cognizant of the potential limitations and pitfalls of intraoperative neurophysiological monitoring and it its necessary that other monitoring tools are needed to prevent the nerve injury during the operation.