Introduction
According to the Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury (SCI) from American Association of Neurological Surgeons/Congress of Neurological Surgeons, administration of methylprednisolone for treatment of acute SCI is not recommended with Level I evidence [1]. This guideline claims that the results of previous reports regarding on the benefit of corticosteroids are mostly inconsistent and have a selection bias, and emphasizes harmful effects of high-dose steroids. However, there still exist controversies regarding on the neurologic recovery after intravenous methylprednisolone (IVMP) in patients with acute SCI, and even more, it is also strongly recommended to administer a high dose of IVMP over 15 minutes, followed by a maintenance dose for 23 hours [2].
Intraoperative neurophysiological monitoring (IONM) is being widely used to prevent postoperative neurologic deterioration [3-5]. However, there has been no previous report of which specifically described the effect of high dose IVMP on the electrical neurophysiological outcomes.
In this case, high dose IVMP was administered promptly after the significant reduction of amplitudes of muscle motor-evoked potentials (mMEPs) during the removal of a cavernous malformation at the T3/4 level. Although the amplitudes of mMEPs did not recover, the amplitude of the direct wave (D-wave) was amplified. This is the first report, which described the effect of IVMP on IONM during spinal cord tumor surgery.
Case Presentation
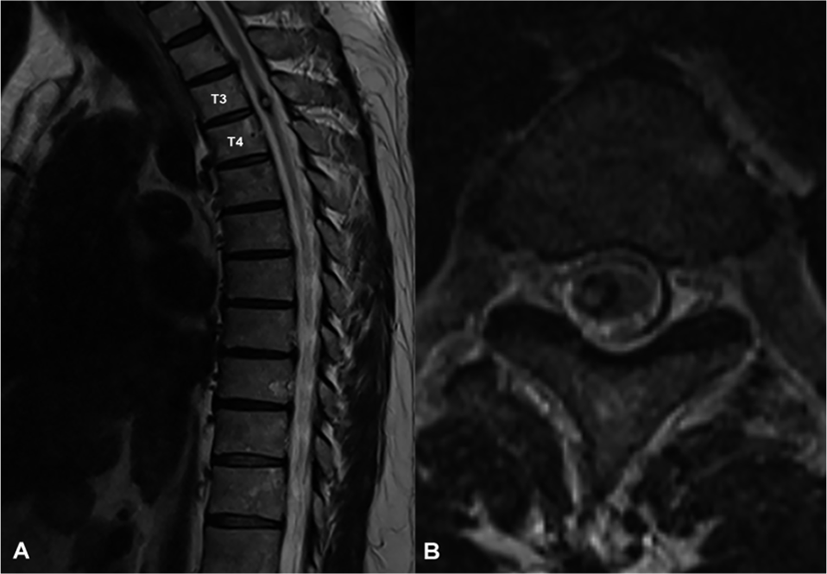
A 58-year-old man who had numbness and progressive weakness in bilateral lower extremities diagnosed with a cavernous malformation at T3/4 level by magnetic resonance image (Fig. 1-A, Fig. 1-B) underwent tumor removal surgery in the Department of Neurosurgery in a tertiary hospital. A day before surgery, the strengths of the 5 key muscles in lower extremities were measured using the Medical Research Council scale based on the International Standards for Neurological Classification of SCI, and the total score of bilateral lower extremities was 44 (Table 1).
| Key muscles | A day before surgery | A day after surgery | 4 months after surgery | |||
|---|---|---|---|---|---|---|
| Rt. | Lt. | Rt. | Lt. | Rt. | Lt. | |
| L2 | 5 | 5 | 5 | 5 | 5 | 5 |
| L3 | 5 | 5 | 5 | 5 | 5 | 5 |
| L4 | 4 | 4 | 5 | 5 | 5 | 5 |
| L5 | 4 | 4 | 4 | 4 | 5 | 5 |
| S1 | 4 | 4 | 4 | 5 | 5 | 5 |
| Total | 22 | 22 | 23 | 24 | 25 | 25 |
Whole surgical procedures were performed under IONM directed by one physiatrist and one technician, including mMEPs, somatosensory evoked potentials (SEPs), and D-waves. The alarm criteria were as follows; more than 50% amplitude reduction or more than 10% latency prolongation of SEPs, more than 80% amplitude reduction of mMEPs, and more than 50% amplitude reduction of D-wave compared to baseline values [3,6].
For transcranial stimulation, C1/C2 and C2/C1 montages were used. Short trains of 6 square-wave stimuli of 0.5 ms pulse width, 3 ms interstimulus intervals, and 2 Hz repetition rate were delivered and the mMEPs were recorded in the bilateral deltoids, abductor pollicis brevis, tibialis anterior (TA), and abductor halluces (AH). With a single pulse of 0.5 ms duration, 150–200 mA intensity, D-waves were recorded in the epidural space at T5 level.
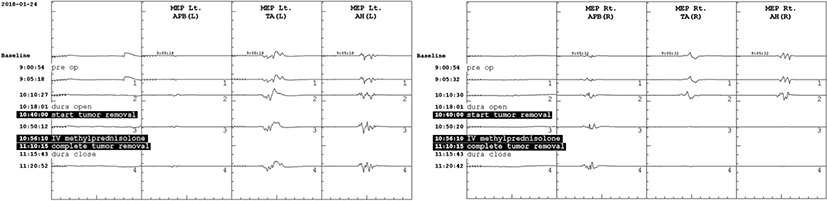
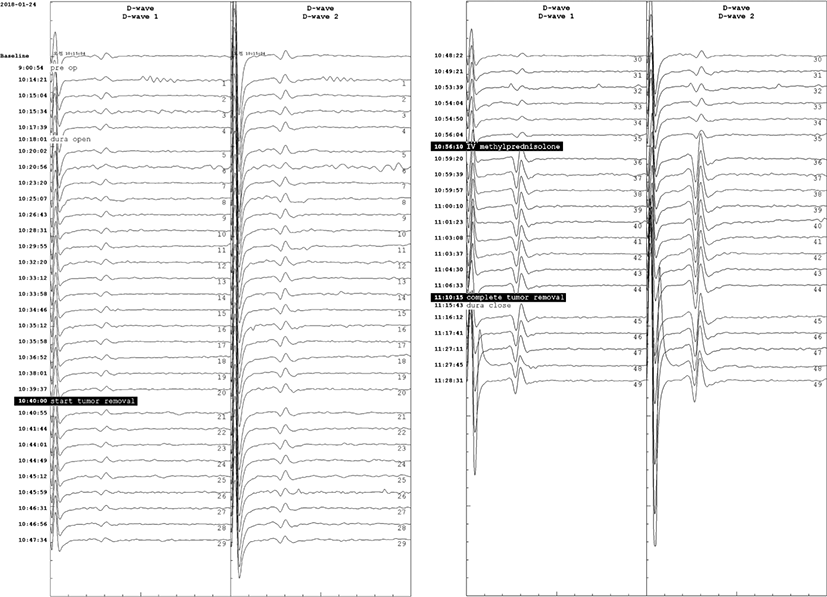
Total intravenous anesthesia was continued with remifentanil and propofol, which have been suggested to be optimal for mMEP monitoring [5]. During the entire operation, the patient’s systolic and diastolic blood pressures were maintained above 95 and 55 mmHg, respectively, in prone position. There were no significant deterioration in SEP or the D-wave amplitude during the tumor resection. However, during the tumor resection, the amplitudes of mMEPs were reduced to 11.4% and 11.3% of the baseline in the right TA and AH, respectively (Fig. 2). At this time, the patient’s blood pressure was 145/82 mmHg and his pulse rate was 103 bpm. Immediately after the alarm, IV SOLU-MEDROL® (methylprednisolone) was administered at a dose of 30 mg/kg over 15 minutes, and maintained at a rate of 5.4 mg/kg/hour during the next 23 hours. While the final amplitudes were 11.2% and 15.3% of the baseline mMEP of the right TA and AH, respectively, the D-waves were abruptly amplified to 597% and 563% immediately after the IVMP administration, and maintained until the end of the operation (Fig. 3).
The total muscle strength score for the bilateral lower extremities was improved to 47 on postoperative day one, and to 50 four months after the surgery (Table 1).
Discussion
Intraoperative monitoring of transcranial electrical stimulation mMEPs provides information regarding neural disruption of the motor pathway during spinal cord tumor surgery, and correlates with early postoperative motor function [3,4]. In this present case, after the spinal cord tumor was removed, mMEPs of right TA and AH muscles significantly reduced beyond alarm criteria. According to the recent concensus, a reduction in MEP amplitude of 80% or more is considered as a minor warning criterion, as it may cause a temporary motor deficit after surgery [3].
As mMEPs can be affected by several factors (anesthesia, position of surgery, stimulation failure, and displacement of electrode), false positive or negative results also can present [3,5]. There was no hypotension or other systemic circulation issues during surgery which also can induce significant changes in evoked potentials.
D-waves have been suggested to be highly associated with long-term postoperative motor outcomes [5]. D-waves recorded in epidural space reflect the direct activation of axons [4,5]. It is known to be more stably recorded than mMEPs, not related to lower motor neurons, less affected by anesthesia [3,5]. Dissection of intramedullary spinal cord tumors may disrupt supportive facilitation systems for lower motor neuron excitability. Damage to this system interferes firing of alpha motor neurons and lead to loss of mMEPs. Several studies on spinal cord tumor surgery have reported significant amplitude reduction of mMEPs without significant changes in D-waves [3,4,7]. Recent reviews recommend co-monitoring of D-waves and mMEPs to reduce false outcomes of IONM in spinal cord surgery, and recommend even if there is a significant reduction in mMEP amplitude, the surgical procedure can be continued if the D-wave does not significantly change, although this may lead to transient weakness in the affected extremity [3-5].
Although there still are controversies in using IVMP, weighing between the reproducibility of efficacy and complication risks, the evidences of the benefits of IVMP in management of acute SCI cannot overlook [2]. Once the complete injury of neural components occurred, the chance of recovery becomes very little. Ahn H et al. has suggested to start IVMP as soon as possible after recognition of iatrogenic SCI during surgery [8]. High-dose IVMP is known to have the neuroprotective effect preventing the secondary axonal injury [8]. The significant electrophysiological recovery was noticed after administration of IVMP in animal SCI models. Young et al. have reported that the administration of 30 mg/kg of IVMP to cats 45 minutes after SCI led to recovery of SEPs 2 to 4 hours after SCI with concurrent rapid reversal of blood flow in the lateral column of the spinal cord 1 to 4 hours after injury, as well as increase in extracellular Ca2+ levels after IVMP administration [9]. De Ley et al. have reported similar results indicating that SEPs recover after administration of 30 mg/kg of IVMP [10]. Lee BH et al. administered 30 mg/kg of IVMP to an experimental group of rat model 15 minutes after spinal cord contusion and observed larger MEP amplitudes and better functional outcomes than the control group [11]. However, these animal studies did not continuously monitor the evoked potentials, so that they only confirmed the postoperative neurophysiological effects of IVMP. There also has been no human study of IONM to determine the effects of perioperative IVMP on iatrogenic SCI during surgery. From this perspective, our study is the first report on the effectiveness of IVMP on IONM results, especially on simultaneous monitoring of mMEPs and D-waves.
In this present study, although the electrophysiological improvement followed by IVMP has not been completely understood, the effects of IVMP may be due to the enhancement of spinal blood flow and the stabilization of the extracellular calcium ion level, as supported by previous work of experimental model [8]. Even considering the significant reduction of mMEPs, the motor score rather improved one day after surgery instead of paralysis, and fully recovered at 4 months after surgery. Although these results may be due to a decompression effect on neural tissue after the tumor removal, considering the significant reduction of mMEPs, the possibility of anti-inflammatory or axonal protective effects of IVMP cannot be excluded.
Conclusion
Our findings suggest that perioperative administration of high-dose IVMP immediately after the detection of the significant reduction of mMEPs amplitudes may improve neural conduction during spinal cord surgery. This is the first report of the large amplification of D-waves immediately after administration of IVMP. From this electrophysiological evidence, we suggest that perioperative administration of high-dose IVMP immediately after the detection of the significant reduction of mMEPs amplitudes may improve neural conduction during spinal cord surgery.







