Introduction
Brainstem auditory evoked potentials (BAEPs) are widely used in monitoring the integrity of longitudinal network from the acoustic nerve to the auditory cortex [1]. Posterior fossa surgery, such as cerebellopontine angle (CPA) tumor removal is where BAEP monitoring has the most value. Tracking changes in amplitudes of wave I, III, V and interpeak intervals of I–III, III–V, and I–V in close temporal relationship to surgical maneuver is imperative throughout the surgery, since it may influence the surgical outcome [2-5]. As a normative rule, the ipsilesional ipsilateral channel wherein stimulation is occurring has the most direct value during monitoring, and the contralateral channel will be used only as a control; the same is true for the contralesional channel. Nevertheless, we herein present a case describing the role of contralesional, contralateral channel in BAEP monitoring during surgical removal of infratentorial extramedullary ependymoma.
Case
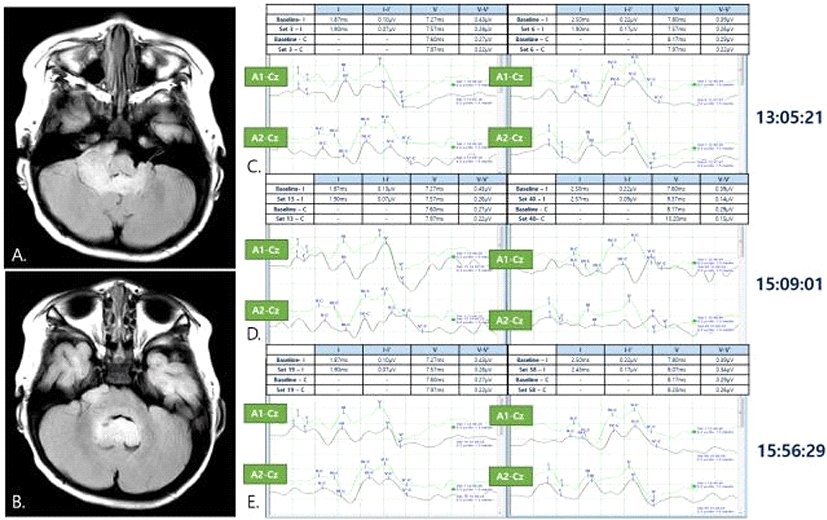
A 12-month old male with no prior medical history presented to the hospital with symptoms of neck hyperextension, difficulty in sitting down, standing up, walking, and crying when laid down in supine position that started five days ago. Brain MRI revealed a 3 × 5 cm cerebellar mass occupying both (more predominantly in the right) CPA and 4th ventricle, which resulted in hydrocephalus (Fig. 1-A, Fig. 1-B). An alleged diagnosis of ependymoma was made and extraventricular drainage (EVD) for obstructive hydrocephalus was performed prior to tumor removal operation to relieve intracranial pressure. Three days after EVD insertion, suboccipital craniotomy and tumor removal operation were done under general anesthesia, with intraoperative neurophysiological monitoring of somatosensory evoked potential (SEP), motor evoked potential (MEP), free-running electromyography (EMG) and BAEP.
Total intravenous anesthesia with midazolam 3 mg, 2% propofol 1,000 mg, and remifentanil 0.5mg were used. Neuromuscular blockade was performed just before intubation with rocuronium 6.4 mg. During the whole operation, train-of-four ratio was kept above 2.
BAEP was obtained by monitoring the two-channels placed in bilateral mastoid processes (A1 and A2, left and right respectively), referenced to Cz when 43.9 Hz auditory stimuli was given by 1.5 msec/div using an in-ear microphone. Bilateral latencies and amplitudes from waves I-V were recorded at baseline.
SEP was obtained from median nerve and posterior tibial nerve. For median nerve SEP, square-wave pulses were administered for 0.2 ms at 15 mA, at frequency of 5.1 Hz. For posterior tibial nerve SEP, square-wave pulses were administered for 0.2 ms at 20 mA, at frequency of 5.1 Hz. Four-channel recording electrodes were placed at C3’, Cz’, C4’, and Fpz. Bilateral N20 latencies were recorded at baseline.
MEP was evoked by delivering train-of-five square- wave transcranial electrical stimuli, each lasting 0.2 ms with an intensity of 400 volts and inter-stimulus interval of 2 ms. The output was recorded in abductor pollicis brevis and abductor digiti quinti muscles for upper extremities and tibialis anterior and abductor hallucis muscles in lower extremities. The baseline MEP threshold was 300 volts.
Free-running EMG was performed in bilateral orbicularis oculi, orbicularis oris, and trapezius muscles.
During surgery, ipsilesional ipsilateral channel in right BAEP already had decreased amplitude from the baseline, but as surgical removal in the right CPA proceeded, wave I, III, and V amplitudes decreased more severely, down to less than 50%. However, the contralesional, contralateral channel demonstrated stable BAEP throughout the surgery (Fig. 1-C, Fig. 1-D, Fig. 1-E). No subsequent intervention other than close observation was performed in response to this change. The tumor was grayish, suitable for suction, and was completely removed from both lateral recesses. Restoration of ipsilesional ipsilateral BAEP was observed after successful removal of ependymoma in the right CPA, 4 minutes after first detection of decreased wave I amplitude. SEP remained stable throughout the surgery. No abnormal neurotonic discharges were observed in free-running EMG.
Discussion
The present case illustrates a promising role of monitoring contrallesional contralateral channel during BAEP monitoring. In cases where ipsilesional ipsilateral BAEP is already attenuated from or after wave I, brainstem pathways are prone to underestimation. The contralesional contralateral BAEP however, has strength in that the brainstem pathways are relatively free of tumor-laden bias of the periphery from acoustic nerve to superior olive. Therefore, in our case, although ipsilesional ipsilateral channel amplitudes were decreased, stable contralesional contralateral BAEP suggested that the brainstem auditory pathway was relatively intact, suggesting that the BAEP fluctuation was probably caused by transient compression and traction of BAEP pathways during surgical manipulation than direct damage to the brainstem.
Functional assessment of brainstem pathway is important because it represents the invasiveness of the tumor. Ependymomas are generally not invasive, but aggressive anaplastic ependymomas can infiltrate the brainstem [6]. Compressive effect by large-growing ependymomas are possible, and obstructive hydrocephalus can cause compression in the longitudinal pathways linking brainstem nuclei to the auditory cortex [7,8]. Contralesional contralateral BAEP monitoring, along with ipsillesional ipsilateral BAEP, remains a useful adjunctive tool in assessing the functional integrity of brainstem through the auditory pathways.
Furthermore, BAEP monitoring can aid in localization of tumor inside the longitudinal axis: intramedullary vs. extramedullary. Imaging is a guiding tool to differentiate the degree of involvement, but form does not always reflect function, and cases with microinvasion to the brainstem had been reported [6]. This information not only can facilitate the quality of surgical performance but also guide to understanding the surgical outcome of the patient.
BAEP is an accurate and reliable indicator of auditory pathway, which can be used in non-invasive fashion, regardless of state of consciousness [9]. BAEP is commonly used for localization of lesion along the auditory pathway by measuring latencies and amplitudes in the stimulation-sided channel (ipsilateral A-Cz). Usually, intraoperative BAEP monitoring adopts the same method of routine BAEP tests, so the waves on the nonstimulation-sided channel may not be of interest practically. However, our case demonstrates that the nonstimulation-sided channel (contralateral A-Cz) at contralesional ear could give some useful information on interpreting the wave changes. Our example emphasizes that a broader interpretation of auditory pathway is possible, using contralesional contralateral channel in BAEP monitoring during posterior fossa tumor surgery.







