Introduction
Spinal vascular malformation is a rare disease entity and can lead to progressive neurological symptoms if not properly treated [1]. Endovascular embolization is one of the treatment options for spinal arteriovenous malformation (AVM) [1]. Since the procedure is performed under general anesthesia, it is not possible to check the patient’s neurological function continuously throughout the procedure. To prevent possible neurological deterioration induced by incidental occlusion of the normal vasculature by the embolic agent, continuous intraoperative neurophysiological monitoring (IONM) is widely performed. The goal of IONM is to detect a possible neurological insult early when it can still be reversed. Several studies reported transient or persistent neurological deterioration followed by significant changes in IONM during spinal vascular interventions [2-4]. Here, we present a case of spinal AVM, where the strategy of endovascular embolization was modulated with the help of IONM and yielded a less worsened outcome.
Case presentation
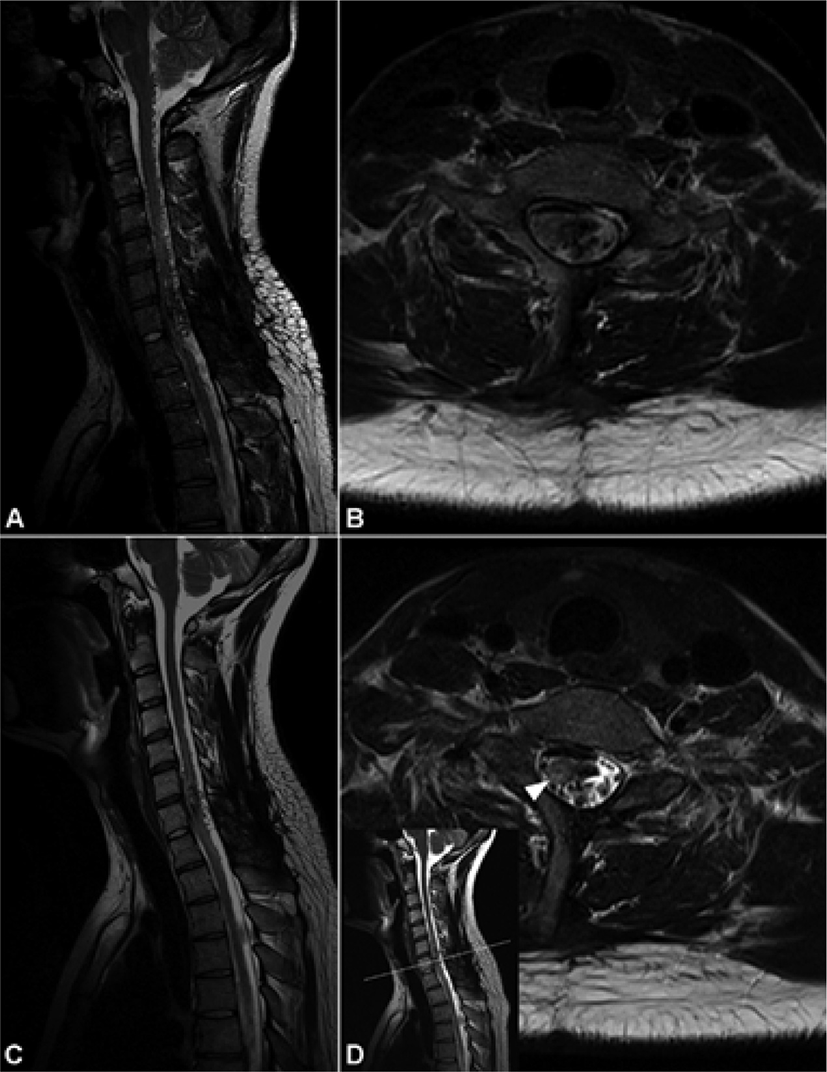
A 24-year-old man who was diagnosed with spinal AVM in 2018 with initial symptoms of headache, posterior neck pain, and loss of consciousness and was admitted to the department of neurosurgery in a tertiary hospital on February 2, 2020. He mainly complained of weakness and tingling sensation of the bilateral lower extremities. He had histories of four times of previous digital subtraction angiographies and selective arterial embolizations; the feeding arteries from the left vertebral artery, left thyrocervical trunk at the level of C6-7, C7-8, C5-T1, and left T6 segmental arteries were selectively embolized. Despite these interventions, magnetic resonance imaging (MRI) showed nidus formation at C7 and flow voids from C7 to C1–2 level in T2-weighted turbo spin echo sequences (Fig. 1-A). Spinal angiography revealed residual spinal AVM with the nidus located in the left posterior aspect of C6–7, with feeding arteries from bilateral T6 segmental arteries and a neuromuscular branch of the left vertebral artery (Fig. 2-A); thus, additional embolization for these three feeding arteries was planned. Before the intervention, a neurological examination was conducted. The Medical Research Council scale for muscle strength was used in measuring the strength of the 10 key muscles of the International Standards for Neurological Classification of Spinal Cord Injury, and the total score was 80. The patient presented slight tingling sensation in bilateral soles. Deep tendon reflexes of the biceps and triceps and knee and ankle jerks were normoreflexive bilaterally. There were no symptoms of bladder and bowel dysfunction. On February 7, 2020, endovascular embolization was percutaneously performed by one experienced radiologist with the assistance of a neurosurgeon. To prevent neurological deterioration that may occur as a complication during the procedure, simultaneous and continuous IONM was planned by a skilled physiatrist and medical technician using the Cascade® IONM system (Cadwell Industries Inc., Kennewick, WA, USA). The somatosensory evoked potentials (SEPs) were obtained by stimulating bilateral median nerves at the wrists and the bilateral tibial nerves at the ankles (duration, 0.2 ms; repetition rate, 5 Hz), recording at C3 (right median), C4 (left median), and Cz (right and left tibial nerves), and referencing FPz according to the international 10–20 EEG system. More than 10% prolongation of latency from the baseline latency or amplitude reduction >50% from the baseline peak-to-peak amplitude were considered as alarm criteria for SEPs. Transcranial electrical stimulation motor evoked potentials (MEPs) were obtained from the bilateral deltoids, abductor pollicis brevis, tibialis anterior (TA), and abductor hallucis brevis (AHB). Train stimuli that consisted of six square-wave stimuli (duration, 0.5 ms; interstimulus interval, 5 ms; repetition rate, 2 Hz; stimulation intensity gradually escalated from 50 mV to 400 mV) were delivered through needle electrodes placed at C1 and C2, according to the international 10–20 EEG system. C1/C2 and C2/C1 montages were used to measure the MEPs of the right and left extremities, respectively. An amplitude reduction >50% compared with the baseline MEPs was considered a significant change.
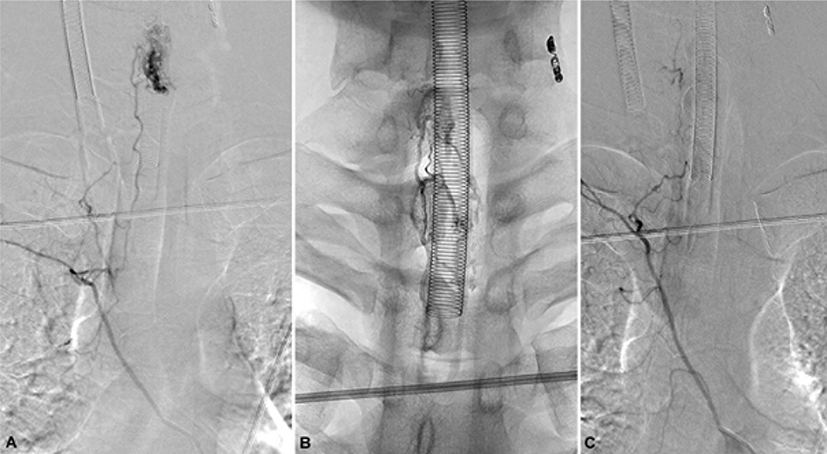
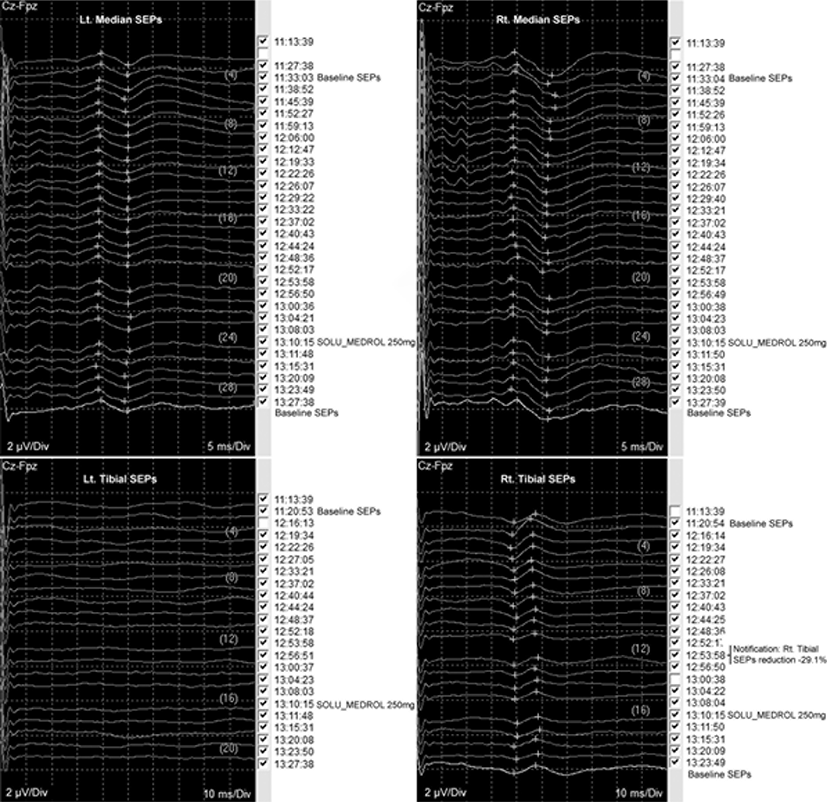
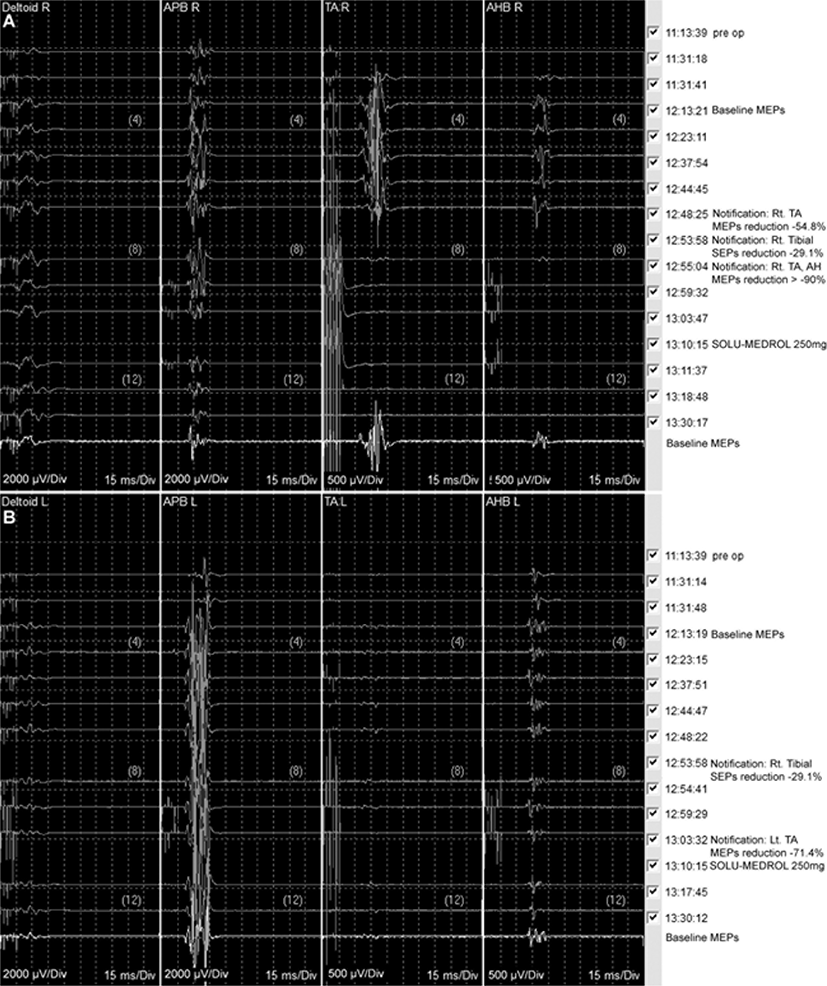
Total intravenous anesthesia was continued with remifentanil and propofol. The baseline waves of bilateral median and right tibial SEPs were stably obtained; however, the waves of the left tibial SEPs were not obtained from the start of monitoring (Fig. 3). Under general anesthesia, a 6-F sheath was placed on the right femoral artery in supine position. A 5-F angiographic catheter was approached after intravenous heparinization (3,000 IU), and a microcatheter (Apollo Onyx Delivery Microcatheter, Ev3, Irvine, CA) was advanced over a microwire (Chikai 10, Asahi Intecc, Nagoya, Japan) to the right segmental artery at the level of the 6th thoracic vertebra (Fig. 2-A). During the approach to the target point via the right 6th thoracic intercostal artery, the SEPs and MEPs showed no significant changes. Embolization was performed using one vial of Onyx® (Ev3 Neurovascular, Irvine, CA) (Fig. 2-B and 2-C). During embolization, the amplitude of MEPs obtained from the right TA abruptly reduced to 58.4% compared to the baseline amplitude (Fig. 4-A), and the interventioner was promptly notified. Nearly at the same time, the amplitude of the right tibial SEPs abruptly reduced to 29.1% from the baseline (Fig. 3). Although, it was a small reduction, which does not meet the alarm criteria, the interventioner was notified since it was an abrupt decrement. Immediately after the alarms, the procedure was discontinued, and intravenous administration of the methylprednisolone was planned. During the preparation of the methylprednisolone administration, the MEP amplitude of the right AHB also reduced to 76.9% compared to the baseline. Then, the waves of the right TA and AHB were nearly flat, the MEPs decreased by >99.0% compared to the baseline. Even after 15 minutes of complete intravenous administration of 250 mg of SOLU-MEDROL® (methylprednisolone), the MEP amplitudes of the right TA and AHB remained decreased by >90% from the baseline, although the amplitude of MEPs were restored approximately 1% and 6% from the right TA and AH, respectively (Fig. 4-A). The MEPs of the left TA also showed decreased amplitude after Onyx embolization, 71.4% from the baseline, but were promptly restored after SOLU-MEDROL® administration (Fig. 4-B). Bilateral median and tibial SEPs did not show significant changes beyond the alarm criteria from the baseline (Fig. 3). Although the original plan to embolize the feeding arteries and veins was not completely achieved, the clinician decided to end the procedure.
Neurological examination performed a day after embolization revealed motor deterioration in the bilateral lower extremities, especially on the right side (Table 1). The total motor score decreased to 69, and the patient could not stand without assistance. Hypoesthesia developed below the bilateral T5 dermatomes. A deep tendon reflex test revealed hyperreflexia in the bilateral knee and ankle jerks, and ankle clonus of 1–2 beats was observed bilaterally. After removal of the indwelling urinary catheter, there was no voiding sensation, and self-voiding was not possible, so clean intermittent catheterization was used for urinary drainage. Anal tone decreased, but voluntary anal contraction was possible during self-defecation. There was no pain in the lower extremities before and after the procedure. On the follow-up MRI performed a day after the procedure, the flow voids at C1–5 nearly disappeared, but the residual nidus at C6–7 was still visualized, and there was focal increment in signal intensity of the right side of the spinal cord at C7, suggesting cord infarction (Fig. 1-C and 1-D). On February 12, 2020, the patient was transferred to the department of rehabilitation medicine and underwent a comprehensive rehabilitation program, including standing and gait training, sensory stimulation, and bladder training with medication. On March 6, 2020, 4 weeks after the procedure, the motor score was restored to 76, and the patient could stand without assistance and walk with bilateral monocanes. Hypoesthesia below T5/T5 and hyperreflexia on both knees and ankles remained. With administration of several medications, he was able to urinate by himself.
Discussion
There have been several case reports of iatrogenic spinal cord infarction that developed during endovascular interventions. Unexpected nontarget artery occlusion due to a retrograde reflux of the embolic agent during embolization leads to spinal cord swelling with functional deterioration [4]. There have been several previous reports describing intracranial dural arteriovenous fistulas with spinal venous drainage manifesting as myelopathy after endovascular embolization with Onyx [3,5,6]. In the present case, there was no evidence of arterial occlusion involving anterior or posterior spinal arteries, and venous thrombosis followed by spinal cord infarction may be the most likely cause of evoked potential deterioration. When Onyx moves to the radial vein of the spinal cord at C7 vertebral level, venous thrombosis and spinal cord infarction can sequentially develop. In this case, other than discontinuing the procedure and administering methylprednisolone, further intervention for removal of Onyx was not performed, since the obvious embolic focus was not detected, while the target point was well occluded.
Onyx is a recently developed liquid embolic agent, which is comprised of ethylene-vinyl alcohol copolymer, and is considered a permanent embolic agent [7]. Although recanalization is possible, it is important to detect the neural insult as early as possible to prevent or minimize iatrogenic post-procedure neurological deterioration. However, as in this case, once the evoked potentials abruptly deteriorated, finding the exact embolic or other problematic focus is difficult, and making a decision whether to remove the embolic agent from the target point is even more difficult. To prevent this type of irreversible situation, other than sole multimodality IONM, several preventive methods have been suggested and performed. Continuous multimodality IONM in combination with provocative testing of target artery has been recommended. Amobarbital, pentobarbital, propofol, lidocaine, etomidate, and methohexital have been used for pre-embolization testing [8,9]. Because of its short-acting property, a bolus of methohexital is used for the provocative test, and if IONM showed significant changes, the test is repeated to verify previous electrophysiological deterioration. If the changes are confirmed, the tested artery is excluded from the target of embolization [10]. However, there still remain issues regarding the accuracy of the test, because methohexital can be shunted away to the smaller and low-flow vessels in response to the AVM’s high flow [10]. Balloon-assisted Onyx embolization can be another preventive method. Recently, a case of Onyx embolization of spinal AVM using a balloon catheter (Scepter C Balloon, MicroVention, Aliso Viejo, Ca, USA) was reported. Although the authors mainly emphasized the superiority of the accessibility of a balloon catheter to the target point compared to that of the classic one, from the perspective of the neurophysiologist, ballooning of the catheter at the target point can be another physical provocative test, which is easily reversed by prompt deballooning.
This case presentation is to emphasize the role of the continuous multimodality IONM in making important decisions during neuroendovascular procedures. The motor function of the patient in the present case could be saved even after the MEPs were nearly flat during the procedure. During IONM, strong and sufficient communication among the medical crew engaged in the whole processes of the procedure is recommended to enable the proper planning for the modification of the procedure, administration of corticosteroids, and postoperative active rehabilitation program.







