Introduction
Intraoperative neurophysiological monitoring (IONM) is a diagnostic tool used to ensure patient safety during surgery [1]. Previous studies have emphasized the importance of IONM in open cranial surgery [2, 3]. In the case of clipping of unruptured intracranial aneurysm (UIA), a complication rate exceeding 10% was reported before IONM was applied [4]. In contrast, the reported complication rate for IONM-applied UIA clipping has been <5% [5].
For IONM during UIA clipping, motor and somatosensory evoked potentials (MEP and SSEP, respectively) are vital diagnostic modalities [6]. MEP mainly reflects the neurophysiological functions of motor pathways and is sensitive to subcortical ischemia [7]. SSEP is used to measure the functional integrity of the sensory pathway; it is relatively more sensitive to cortical ischemia and is also associated with overall cerebral perfusion [8]. Several studies have suggested the need for the complementary interpretation of MEP and SSEP on IONM, as well as the consideration of various perioperative factors [9,10].
In unilateral open cranial surgery, the change in evoked potential (EP) occurs mainly on the counter side of the involved cerebral hemisphere–the lesional side–due to the decussation of the motor and sensory pathways [11]. However, intraoperative neurophysiologists and surgeons often encounter non-lesional side EP deterioration with normal lesional side EP during surgery. To our knowledge, there have been no reports or proposed decision protocols for non-lesional side EP deterioration during unilateral open cranial surgery.
In this context, this study investigated a case series showing non-lesional side EP deterioration that appeared first without lesional side EP change during UIA clipping surgery. Furthermore, we discussed how neurophysiologists or surgeons might make decisions regarding non-lesional side EP deterioration during surgery.
Materials and Methods
This single-center, retrospective study targeted patients who underwent single UIA clipping with IONM between March 2017 and December 2021. Since we intended to select only lateralized UIA clipping based on the aneurysm location, we included only cases corresponding to UIA located in the middle cerebral artery bifurcation (MCAB), middle cerebral artery (MCA), anterior choroidal artery, and internal carotid artery (ICA). The exclusion criteria for this study were: 1) UIA clipping located in the anterior cerebral, anterior communicating, or posterior communicating arteries; 2) multiple UIA clipping; 3) clipping for ruptured intracranial aneurysm; 4) the use of other treatments such as coiling or bypass in addition to clipping; and 5) concomitant intracranial pathologies such as infection, tumor, or vascular malformation.
The operating duration was defined as the time from induction to the end of the surgery. Aneurysm location and size were identified based on preoperative digital subtraction angiography findings. Postoperative neurologic deficit (PND) was defined as an increase in modified Rankin scale grade, which was measured before, immediately after, and 1 month after surgery. Radiologic findings were interpreted based on brain computed tomography (CT) taken within 24 h or brain magnetic resonance images taken within 48 h after surgery.
This study was reviewed and approved by the Institutional Review Board of our hospital (Approval number: PSSH0475-202202-HR-008-01). Informed consent was not required due to the retrospective design of this study. This study was conducted in compliance with the principles of the Declaration of Helsinki.
All patients underwent IONM using an XLTEK Protektor 32 system (Natus Medical, Oakville, ON, Canada). For UIA clipping, we applied monitoring modalities including MEP, SSEP, and electroencephalogram.
MEP was recorded in the flexor carpi radialis and abductor pollicis brevis muscles of both upper extremities and the tibialis anterior and abductor hallucis muscles of both lower extremities. MEP stimulation was performed in a repetitive and five-pulse train method with an intensity of 200–400 V. The pulse duration was 0.05 ms and the interstimulus interval was 1–4 ms. We set the filter range to 10–3,000 Hz. Transcranial electrical stimulation with needle electrodes was used, with the electrodes placed at C1 and C2 according to the international 10–20 system.
To obtain SSEP, we applied square-wave 0.3-ms electrical pulses at a frequency of 1.75 Hz with stimulation intensities of 25 mA for median SSEP and 30 mA for tibial SSEP. The stimulation site was the palmar side of the wrist for median SSEP and just proximal to the lateral malleolus of the ankle for tibial SSEP. For the recordings, we placed the electrodes at C3’, C4’, Cz, and C5 according to the international 10–20 system, with the reference electrode at Fpz. We set the filter range to 30–1,000 Hz.
Baseline MEP and SSEP values were defined as the recordings obtained just before the dura opening. The warning criteria were a ≥ 50% decrease in MEP amplitude, a ≥ 50% decrease in SSEP amplitude, and a ≥ 10% SSEP latency delay compared to the baseline data [12]. We classified patients who showed deterioration corresponding to the EP warning criteria during surgery into groups with lesional side EP change first (lesional EP group) and non-lesional side EP change first (non-lesional EP group).
In the case of EP deterioration, we performed rescue interventions according to our protocol, as described in detail previously [13]. The basic principles of rescue intervention are fast evaluation and a multidisciplinary approach when an EP change meets the warning criteria [5,14,15]. The surgeon performs cause identification in the surgical field, and the neurophysiologist checks the possibility of technical problems while maintaining continuous EP monitoring. At the same time, the anesthesiologist measures a patient’s blood pressure, body temperature, and anesthetic regimen changes. In the case of a single non-lesional side EP deterioration, we performed close monitoring without rescue intervention. However, as soon as two or more non-lesional side or at least one lesional side EP deterioration was detected on close monitoring, the operation was stopped, and a rescue intervention was performed. We categorized suspected events causing EP deterioration into dura opening, temporary clipping (TC), permanent clipping (PC), and uncertain etiologies.
All patients underwent total intravenous anesthesia. For induction, 3–5 mg/mL of propofol and 3–5 ng/mL of remifentanil were administered. Then, 2.5–3.5 mg/mL of propofol and 2.5–4.5 ng/mL of remifentanil were continuously infused. Before intubation, a single bolus of rocuronium bromide (0.4–0.5 mg/kg) was used and not during surgery. Likewise, no inhalation agent was used during surgery.
Continuous variables were expressed as medians (interquartile range), with Mann–Whitney U tests used for comparative analyses between the two groups. Categorical variables were expressed as frequencies (proportion), with the chi-squared (trend) or Fisher’s exact tests performed for comparative analyses between the two groups. GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, CA, USA) was used for statistical analyses.
Results
During the study period, 315 patients were finally enrolled. Twenty-seven patients (8.6%) presented with one or more EP deterioration events that met the warning criterion during surgery; among them, nine patients showed non-lesional side EP change first (two males and seven females).
Comparative analyses revealed that the group where non-lesional EP group was relatively younger, at 57 years (52.5–70.5 years). TC was applied in seven patients (38.9%) in the lesional EP group, a higher ratio compared to the other group. In the non-lesional EP group, one patient (12.5%) showed transient PND. On the other hand, five patients (27.8%) showed PND in the lesional EP group, with one patient showing PND persisting more than one month after surgery. However, statistical significance was not confirmed between the two groups for all variables (Table 1).
Table 2 summarizes the patients in the non-lesional EP group. Five patients showed MEP changes, and four patients showed SSEP changes. Two patients had right-sided UIA. Seven patients had MCAB UIA, one had MCA UIA, and one had ICA UIA. All patients did not show significant collateral circulation on preoperative digital subtraction angiography.
IONM: intraoperative neurophysiological monitoring; mRS: modified Rankin scale; TC: temporary clipping; F: female; MCA: middle cerebral artery; TA: tibialis anterior; MEP: motor evoked potentials; MCAB: middle cerebral artery bifurcation; SSEP: somatosensory evoked potentials; ICA: internal carotid artery; PC: permanent clipping; FCR: flexor carpi radialis; APB: abductor pollicis brevis; SAH: subarachnoid hemorrhage; M: male; AH: abductor hallucis.
Five patients showed EP deterioration after PC. Others showed EP deterioration after TC (one patient) and dura opening (three patients). Four patients showed only a single non-lesional side EP deterioration, and one patient had two non-lesional side MEP deteriorations; these five patients who did not present lesional side EP change did not have PND or radiologic complications. One case showed a transition to lesional side EP deterioration after the non-lesional side EP deterioration was recovered; she did not show PND.
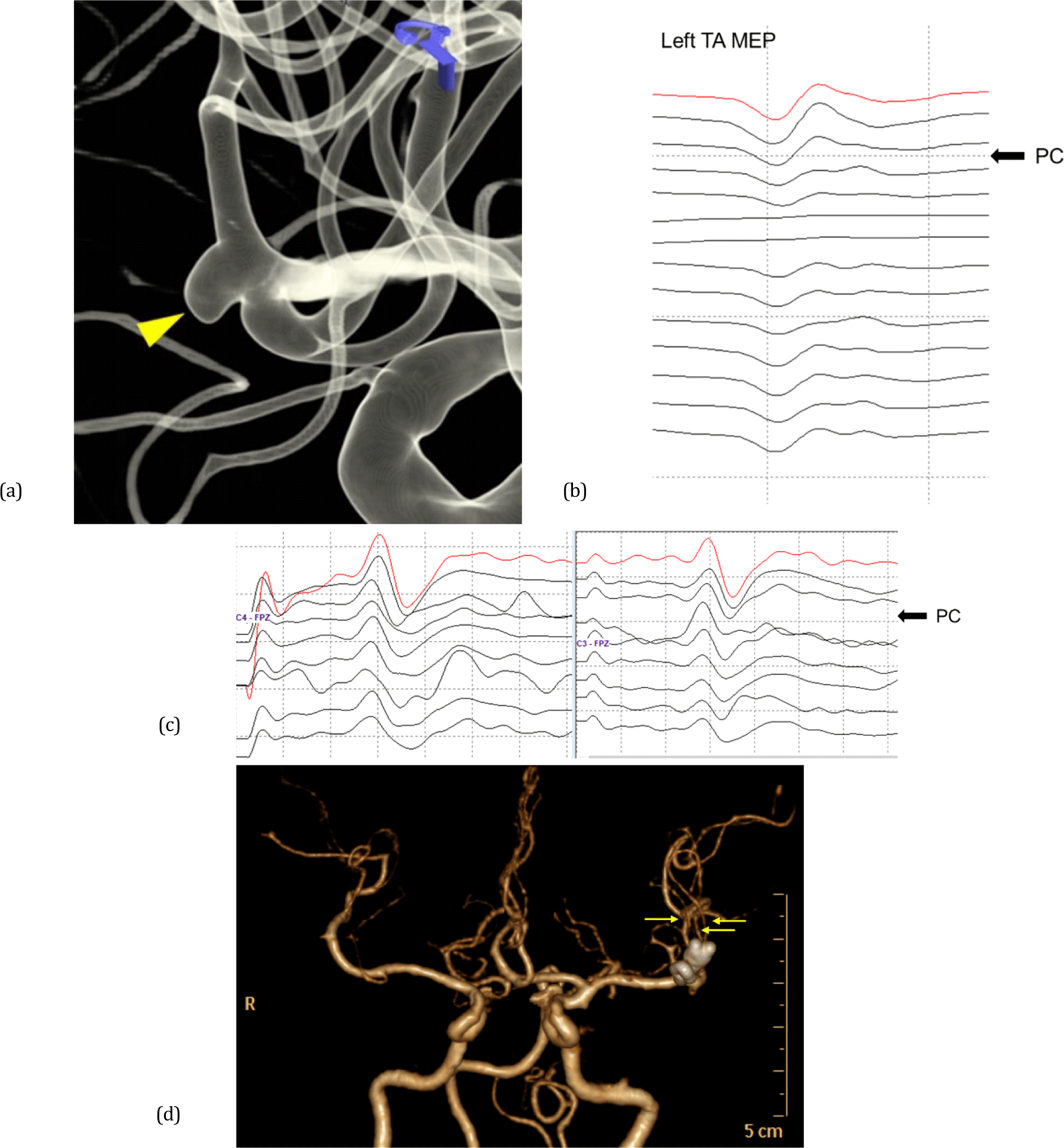
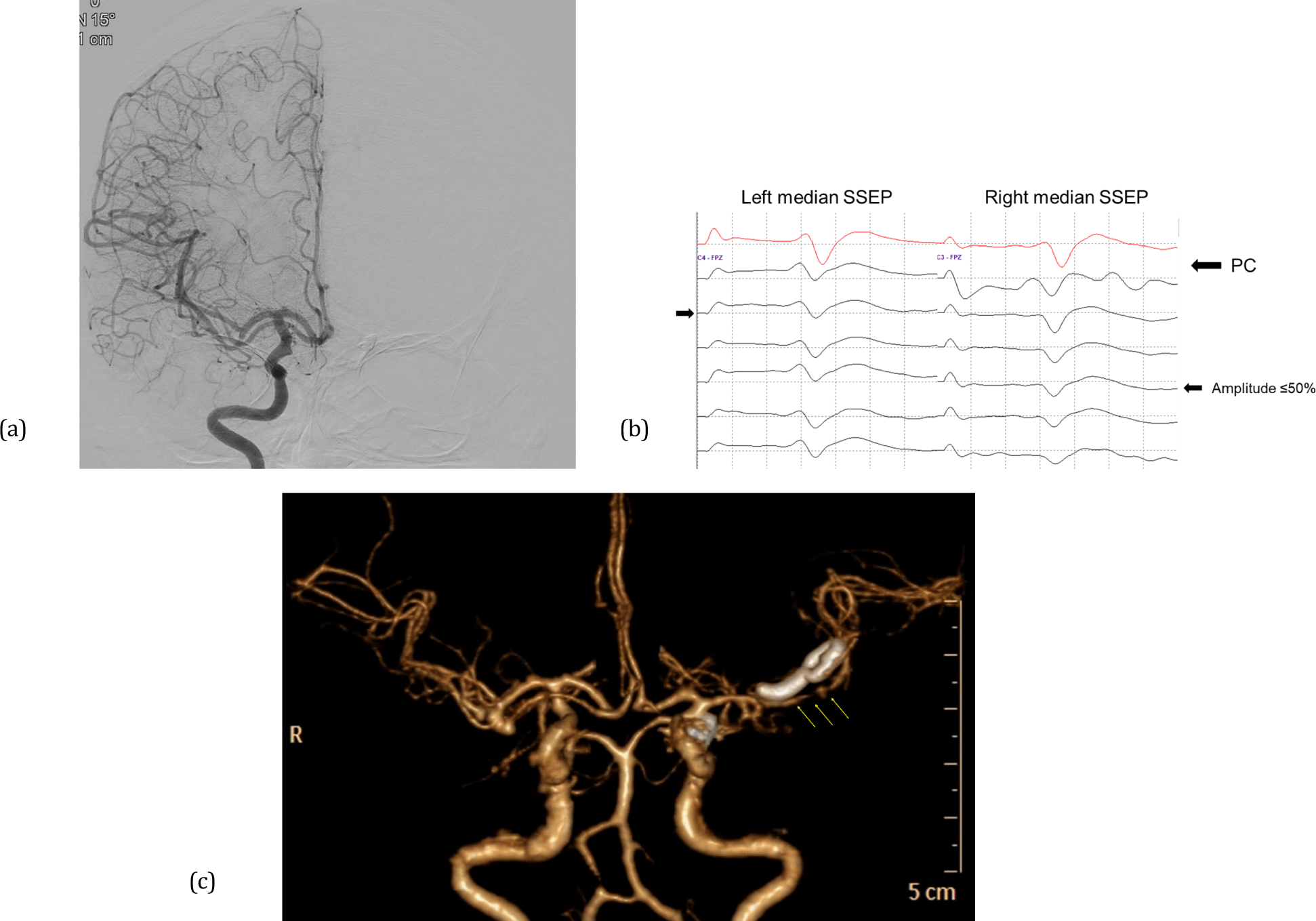
There were three notable cases that presented a transition from the non-lesional side to bilateral EP deterioration. One showed a decrease in the amplitude of the non-lesional side MEPs after PC application, followed by a diffuse reduction in the amplitude of the lesional side EP. The patient showed subarachnoid hemorrhage on postoperative CT findings and complained of bilateral lower extremity weakness. Conservative treatment was applied, and finally, the patient was fully recovered one month after surgery. Meanwhile, two patients had no PND but showed vasospasm on postoperative brain CT. In one case, the non-lesional side MEP decreased first, followed by the bilateral median SSEP deterioration (Fig. 1). The other case showed the non-lesional median SSEP amplitude reduction first, and then the lesional side median SSEP amplitude was also decreased (Fig. 2). There was no PND in both cases.
Discussion
This study investigated a case series of patients that initially presented non-lesional side EP deterioration without lesional side EP changes in a single UIA clipping surgery. This study is significant because it is the first to target only patients who showed non-lesional side EP change first on IONM during lateralized unilateral open cranial surgery.
More than 30% of patients with intraoperative EP deterioration showed non-lesional side changes first. Among them, all patients who transitioned to bilateral EP changes experienced transient PND or radiologic complications. In contrast, none of the patients with only non-lesional side EP changes had any complications. Our results may provide some hints to neurophysiologists and surgeons on the intraoperative decision to take in patients with initial non-lesional side EP deterioration. Our results confirmed that the ratio of initial non-lesional side EP change was not low compared to the overall EP deterioration, suggesting that neurophysiologists and surgeons should pay attention to non-lesional side changes even if there is no EP change on the opposite side. In addition, we suggest that close monitoring should be maintained, recognizing the approximately 50% probability of an actual warning signal occurring on the lesional side after a single non-lesional side EP deterioration.
We did not perform rescue interventions in cases with only a single non-lesional side EP change. However, we immediately stopped the operation and applied the rescue intervention when two or more non-lesional side EP deterioration or at least one lesional side EP deterioration was detected. Since IONM is a diagnostic modality used to maximize patient safety, neurophysiologists should sensitively respond to warning signs. However, the immediate cessation of surgery for all warning signs makes surgeons irritable and may harm patients by excessively increasing the total operation time. Therefore, a balanced decision between neurophysiologists and surgeons is required, and a multidisciplinary approach is essential. Our findings can serve as a reference for determining an interdisciplinary decision protocol for non-lesional side EP deterioration without lesional side EP changes.
The mechanisms of non-lesional side EP changes that occur alone during unilateral open cranial surgery have not yet been elucidated. However, we can infer these mechanisms from the results of previous studies. The non-lesional side motor response has been explained as a cause of the crossover phenomenon of the motor pathway or non-decussated corticofugal fibers [16,17]. Gonzalez et al. [18] reported the crossover phenomenon of transcranial MEP on IONM during open cranial surgery. This study suggests that the crossover phenomenon leads to false-negative results and may be the basis for explaining who two of our patients first showed non-lesional side MEP amplitude decrease and then lesional side MEP amplitude decrease; one showed PND, and one presented cerebral vasospasm on postoperative brain CT. In addition, studies have explained the non-lesional side MEP response as the neurotransmission of corticoreticulospinal or corticopropriospinal pathways according to the hyperexcitability of the premotor area [19]. Conversely, we inferred that these tracts could also be involved in non-lesional side MEP deterioration. Previous studies explained non-lesional side SSEP recording as a cause of transcallosal secondary activation or a short-latency response [20,21]. Noachtar et al. [22] analyzed non-lesional side median SSEP, which has a different spatial distribution from that of lesional side SSEP, reporting that the non-lesional side median SSEP exhibited a relatively low amplitude and a wide range of latency. In addition, spinoreticular, spinomesencephalic, spinocerebellar, and spinocervical tracts have been suggested as uncrossed non-lesional side afferent pathways [23].
We observed three cases of EP deterioration after dura opening in the non-lesional EP group. Cerebrospinal fluid drainage or brain shrinkage after dura open is known as one of the significant causes of EP change during open cranial surgeries; these cause changes in the electrical field configuration of transcranial electrical stimulation [24,25]. Therefore, we can infer dura opening is one of the causes of non-lesional EP change. This has nothing to do with brain parenchymal damage. In fact, in our case series, all non-lesional EP deterioration after dura opening did not progress to the lesional side, and no PND occurred.
This study has several limitations. First, this was a single-center, retrospective study with a limited sample size. Therefore, the results cannot be generalized. Moreover, due to the small number of patients, we could not prove a significant difference between non-lesional and lesional EP groups. A study with a large sample size based on multiple centers is required. Additionally, our outcome measure was based on the modified Rankin scale, a functional indicator that does not easily reflect non-motor symptoms. To accurately detect PND, a study design that considers various clinical symptoms should be considered.
Conclusion
Although the results of this study are not sufficient to conclude, non-lesional side EP deterioration that appeared first without lesional side EP change during the lateralized single UIA clipping should not be ignored. An immediate response is required in cases where the single non-lesional side EP deterioration transits to the lesional side. Our findings provide a basis for neurophysiologists and surgeons to make decisions regarding non-lesional side EP deterioration during open cranial surgeries. However, further multi- center, large-scale studies are needed.







