Introduction
Recurrent laryngeal nerve (RLN) injury is one of the most frequent and serious iatrogenic complications of anterior cervical discectomy and fusion (ACDF), with reported incidences ranging from 0.2% to 24.2% [1]. RLN injury can result in dysphonia, dysphagia, or aspiration, which can negatively impact the quality of life while increasing both morbidity and mortality [2,3]. The laryngeal adductor reflex (LAR) is an involuntary protective airway response to stimuli in the laryngeal mucosa. Intraoperative LAR monitoring is a newly developed technique to assess and monitor RLN, mainly during neck surgery (thyroid and parathyroid surgery) [4]. However, the role of LAR monitoring in ACDF surgery remains poorly explored. Here, we describe a case in which intraoperative LAR monitoring was successfully performed during ACDF.
Case Report
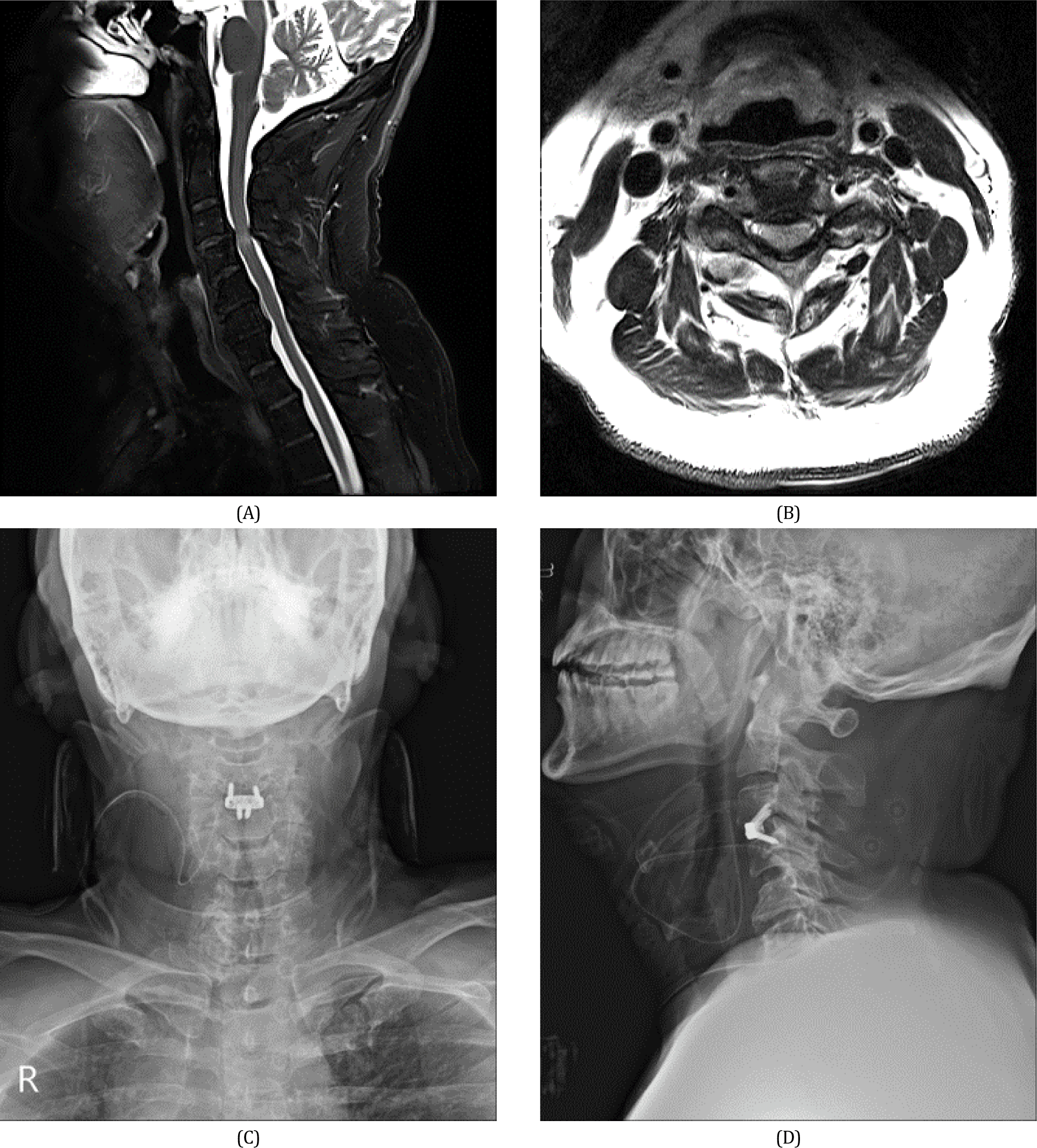
A 59-year-old man with a history of neck pain and radiating pain in his bilateral shoulders and upper extremities for 1 year visited our hospital owing to aggravated neck pain for 2 weeks before admission. On the day of admission, neurological examination revealed weakness in the upper extremities (Medical Research Council grade 4/5), reduced tactile and pain sensations over the C5–C8 dermatomes, and bilateral Lhermitte’s sign. Magnetic resonance imaging was obtained, which demonstrated central canal stenosis with cord compression with signal change at C3–C4 and neural foraminal narrowing with disc protrusion at C3–C4 (Fig. 1-A, Fig. 1-B). We planned to perform ACDF and place the intervertebral cage at the level of the C3–C4 with intraoperative neurophysiological monitoring.
The patient was placed in a supine position under general anesthesia. Total intravenous anesthesia was induced and maintained using the target-controlled infusion of propofol (2–8 μg/mL) and remifentanil (2–10 ng/mL). Following anesthesia induction, tracheal intubation was facilitated using Esmeron (50 mg), without a maintenance dose. The patient was intubated with a NIM TriVantage EMG endotracheal tube (Medtronic Xomed, Jacksonville, FL, USA) to monitor the LAR. Intubation was performed using a video laryngoscope to ensure optimal tube positioning and direct contact between the tube-based surface electrodes and the mucosa of the vocal cords. To prevent displacement, the oropharynx was packed with rolled gauze, and the tube was fixed to the chin with tape. The LAR was elicited by an independent single electrical stimulus to the right or left side of the laryngeal mucosa (duration = 0.1 ms, intensity = 5 mA) using endotracheal tube-based surface electrodes. Contralateral LAR responses were recorded using endotracheal tube-based surface electrodes located opposite the stimulating side. After placement of the endotracheal tube, robust and reproducible LAR responses were bilaterally elicited. We further monitored intraoperative somatosensory evoked potentials (SSEPs), transcranial electrical motor evoked potentials (MEPs), and continuous electromyography (EMG), in addition to LAR. Intraoperative neurophysiological monitoring was performed using an MEE-1000 IOM/EP intraoperative monitoring system (Nihon Kohden, Tokyo, Japan). MEPs were recorded on the bilateral limb muscles (deltoid, triceps, abductor pollicis brevis, vastus lateralis, tibialis anterior, and abductor hallucis). A train of five square-wave pulse stimuli (each pulse duration = 0.2 ms, inter- stimulus interval = 2 ms, intensity = 200 V) was delivered between the two electrodes placed at C3/C4. We further recorded SSEPs using needle electrodes placed on the scalp in the C3’, C4’, and Cz’ against a reference electrode at Fpz. To obtain SSEPs, square-wave electrical stimuli (pulse duration = 0.3 ms, intensity = 10 mA for the median nerve /20 mA for the tibial nerve, frequency = 4.7 Hz) were delivered. Spontaneous EMG activity was monitored using the same electrodes used for muscle MEP recordings.
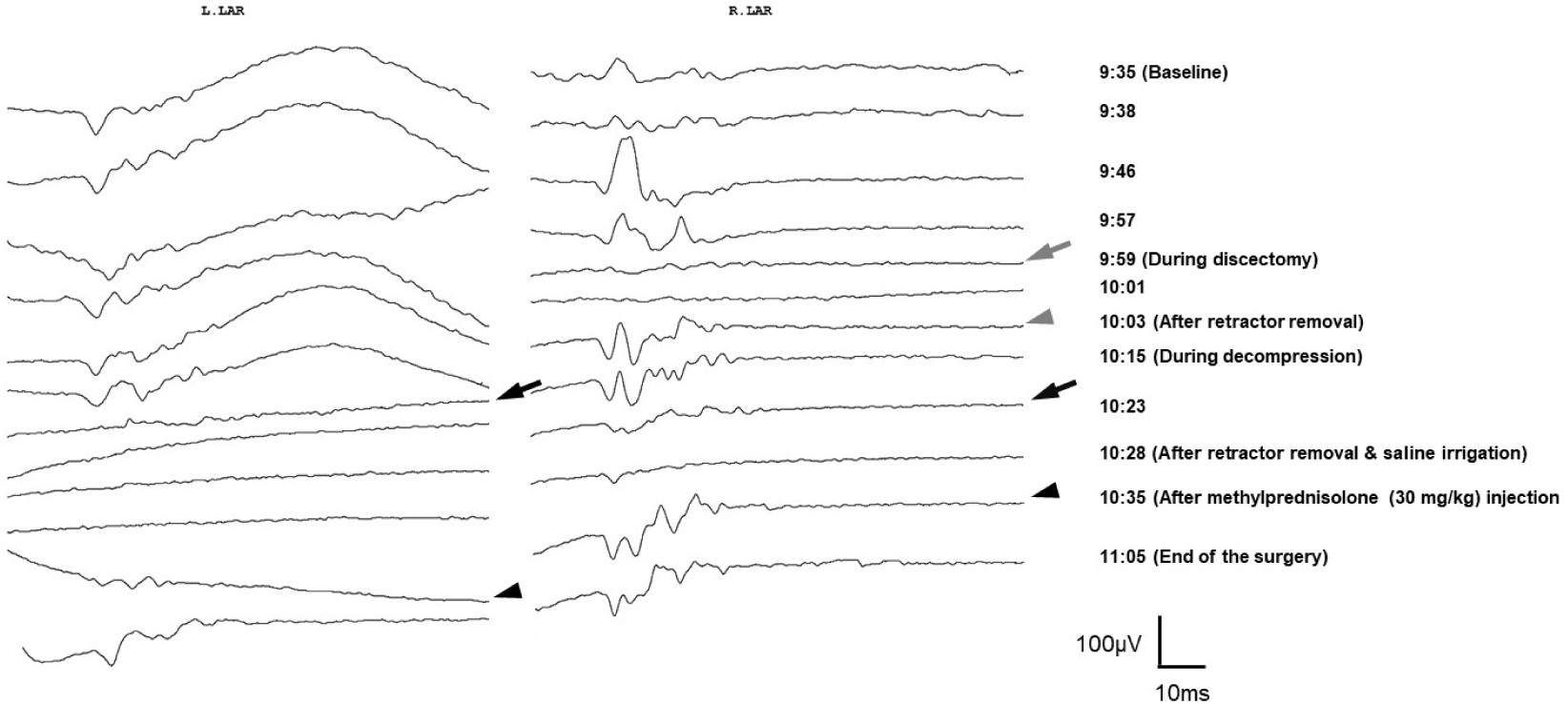
ACDF were performed, with concurrent intraoperative neurophysiological monitoring. The baseline LAR was successfully obtained bilaterally. During the discectomy, the LAR abruptly disappeared on the right side, returning immediately after retractor removal. During decompression of the dural sac, the left and right LARs disappeared in sequence. Other monitoring tools showed no significant changes during LAR loss. Immediately after notification of LAR loss, the surgeon temporarily stopped the surgery, irrigated the surgical field, and released the retractors. Additionally, a single intravenous bolus of methylprednisolone (30 mg/kg) was administered. The bilateral LARs returned after a few minutes and remained stable until the end of the surgery (Fig. 2).
There were no peri or postoperative complications related to intraoperative LAR monitoring, such as ventilatory failure or tracheal injury. One month after surgery, the patient reported that the previous pain radiating to his upper extremity had already diminished, and an improvement in sensory and motor functions was observed. On radiological evaluation, we observed that the cage was still in the right place (Fig. 1-C, Fig. 1-D). The patient showed no evidence of dysphagia or dysphonia until 6 months of follow- up after surgery.
Discussion
The LAR is a brainstem-modulated involuntary response of the thyroarytenoid muscle to various stimuli applied to the laryngeal mucosa [5]. The major function of this reflex is the protection of the larynx, particularly in preventing foreign matter from entering the airway. Impairment of this reflex can lead to dysphonia, dysphagia, or aspiration, which may reduce the quality of life and worsen morbidity or mortality [5,6]. Therefore, assessing and monitoring the integrity of the LAR is important during the surgery linked to the risk of injury to the vagus nerve or lower brainstem structures. The afferent (sensory) nerve of the LAR is the superior laryngeal nerve (SLN), and the efferent (motor) nerve is the RLN, both of which branch off the vagus nerve. Afferent sensation from laryngeal mucosa mechanoreceptors travels via the internal branch of the SLN to the nucleus tractus solitarius which send inputs via interneuronal pathways to the ipsilateral nucleus ambiguus. The motor neurons within the nucleus ambiguus then project to the RLN for the thyroarytenoid muscle contraction [4,5,7]. Previously, the response of the RLN was recorded as the compound motor action potential from the thyroarytenoid muscle using endotracheal tube- based surface electrodes. This can be performed by ipsilateral stimulation of the RLN using a handheld nerve stimulator or clip electrodes positioned on the vagus nerve [8,9]. Recently, Sinclair et al. introduced a method to assess and monitor the integrity of the laryngeal and vagus nerves via LAR using endotracheal tube-based surface electrodes alone to stimulate and record responses [4]. This new method has several advantages over the previous ones that have the potential to broaden the use of the technique: 1) The main advantage is its simplicity and ease of application. It requires an EMG endotracheal tube alone without placement of additional neural probes. 2) It can continuously monitor the nerve integrity without actual nerve exposure. 3) As it can monitor the entire vagal reflex arc, including the laryngeal, vagal, and brainstem structures, its use applies to any surgery accompanying the risk of injury to these structures.
ACDF are one of the most common operative interventions for degenerative cervical spine diseases, and their demand is expected to increase as the population ages. Although the approach to the anterior cervical spine is technically simple, it requires dissection of important neighboring anatomical structures, including the RLN. Injury to the nerve may occur owing to direct mechanical trauma or indirectly owing to traction or compression. Injury to the RLN is a frequent and potentially dangerous complication of ACDF surgery, which may lead to hoarseness, dysphagia, and subsequent aspiration [1–3]. This condition may be reversible or irreversible, depending on the severity of the nerve injury. Therefore, intraoperative assessment and monitoring of the integrity of the RLN during ACDF are important for patient safety and the quality of care. Over the past decade, intraoperative LAR has been used in neck surgery and has shown promising results regarding vagus nerve and RLN monitoring and prediction of postoperative complications [10]. However, the role of LAR monitoring in ACDF surgery remains poorly investigated. Recently, a case series of patients undergoing ACDF with intraoperative LAR monitoring was reported in a larger series, demonstrating the feasibility of this technique [4,7]. However, these reports did not describe the detailed data or the usefulness of intraoperative LAR monitoring during ACDF. In the present case, the intraoperative LAR disappeared transiently during surgery, but the waveform recovered close to the baseline level before the end of surgery. The patient did not exhibit postoperative dysphonia or dysphagia. We thus inferred that intraoperative feedback and temporary halts in surgical manipulation may prevent or mitigate irreversible neurological injury that may have led to the permanent postoperative deterioration of LAR.
This new methodology has certain limitations that must be overcome to allow common usage. The first limitation relates to correct endotracheal tube placement and tube displacement during surgery. This technique uses endotracheal tube-based surface electrodes for both stimulation and recording; thus, it is highly sensitive to changes in the position of the endotracheal tube. We sought to overcome this limitation by applying the following procedure: 1) Correct tube positioning was successfully achieved under direct visualization using a video laryngoscope. 2) The tube was placed at the center of the oral cavity and fixed with tape to the chin. 3) The oropharynx was packed with rolled gauze to reduce tube movement. Second, there are no normative data or established alarm criteria for intraoperative LAR monitoring that correlate with clinical prognosis and postoperative complications. Further studies are needed on the interpretation of intraoperative LAR changes and identification of significant changes predicting clinical prognosis and postoperative complications.
Although the patient had no symptoms or signs of the RLN injury after ACDF surgery, one limitation of our case is that we did not rule out the possibility of subclinical conditions. A future study design with a routine postoperative laryngoscopy in all patients undergoing ACDF surgery can help to ensure the validity and reliability of the results.
In conclusion, if intraoperative LAR monitoring is used properly in ACDF surgery, it could be expected to reduce RLN injury and ultimately improve postoperative prognosis. However, there are still challenges to overcome to achieve successful clinical application, and future studies with a large number of cases are needed to explore its validity and reliability.







