Introduction
The improvement of intraoperative neurophysiological monitoring (IONM) is one of the major contributors to increasing the safety and feasibility of brainstem surgery. Neurophysiological monitoring of each lower cranial nerve (LCN) can prevent the risk of postoperative complications such as dysphagia, dysarthria, and hoarseness [1–3]. Herein, we demonstrate the typical case of cerebellopontine angle tumor resection surgery with cranial nerve monitoring presenting abrupt decrement of the motor evoked potential (MEP) of the upper trapezius (CN XI) and the tongue (CN XII), followed by pharyngeal dysphagia.
Case Report
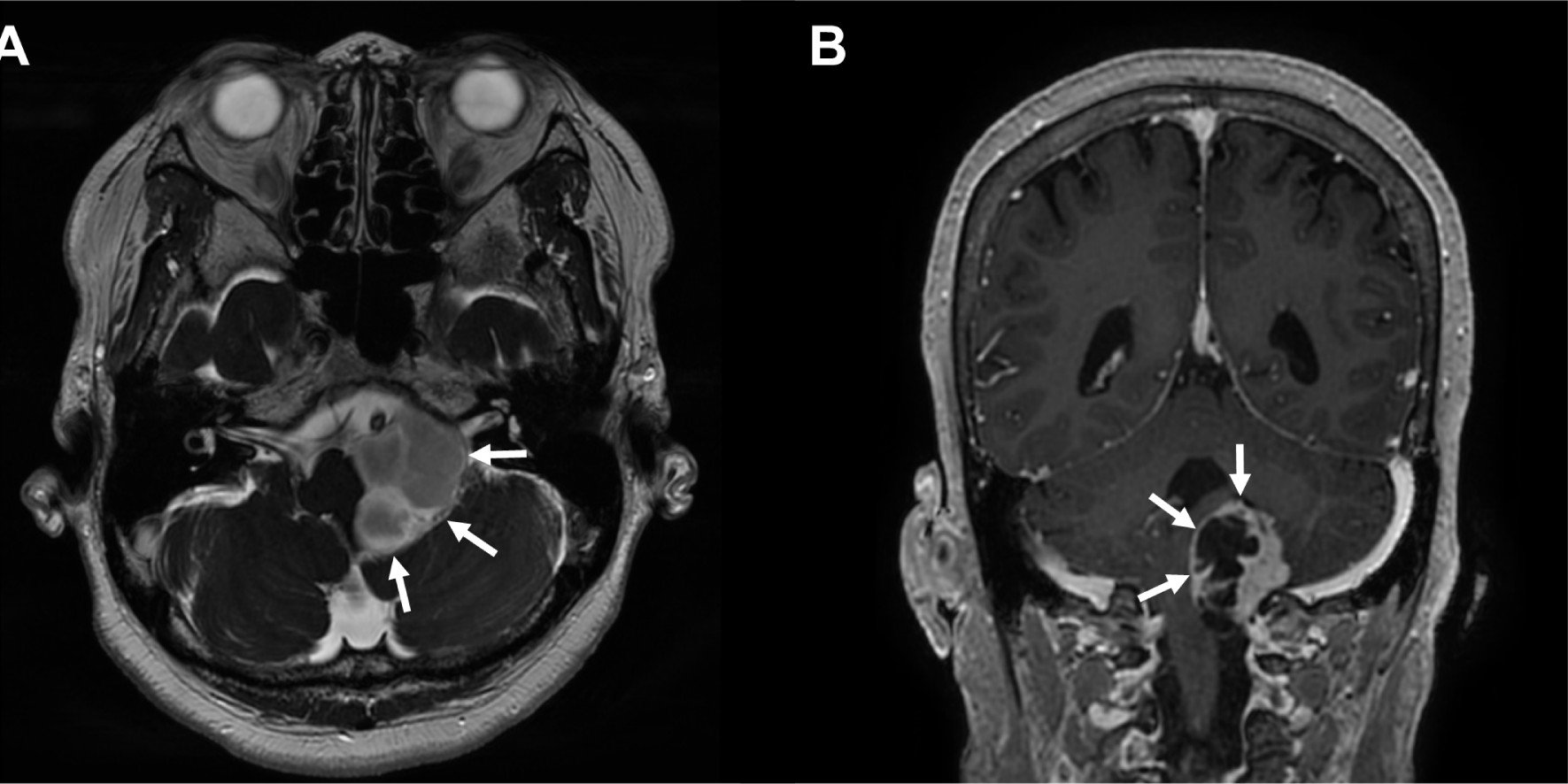
A 67-year-old female suffered from a sustained headache for over 1 year. The patient also had a symptom of frequent aspiration tendency. The brain magnetic resonance image (MRI) revealed a homogeneously enhancing mass on the T2-weighted images with inner multiple cystic portions at the left cerebellopontine angle (Fig. 1-A). The mass compressed the brainstem, left cerebellum, and even spinal cord caudally (Fig. 1-B). There were no other definite neurological abnormalities such as extraocular movement limitation, facial palsy or hypesthesia, hearing impairment, abnormal gag reflex, and deviation of the uvula and tongue in the preoperative cranial nerve examination. The patient went through midline suboccipital craniotomy and tumor removal. The final pathology revealed that the cystic mass was a schwannoma (WHO grade 1).
For the IONM of cranial nerves XI-XII, transcranial MEP and spontaneous electromyography (s-EMG) were recorded from the bilateral upper trapezius and left tongue muscles. For transcranial electrical stimulation, needle electrodes were inserted into the scalp at C3 and C4 following the International 10–20 System. A train of six pulse stimuli (biphasic square) was applied by a commercialized transcortical stimulator, with a frequency of 1 Hz and a stimulus interval of 1–2 ms. The mean stimulation intensity was 350 V. Additionally, the brainstem auditory evoked potential (BAEP) at the left side, transcranial MEP, and s-EMG were recorded at bilateral orbicularis oculi, orbicularis oris, thenar, and abductor hallucis muscles. The somatosensory evoked potential (SSEP) was monitored at the bilateral median and posterior tibial nerves. General anesthesia was induced and maintained by continuous infusion of propofol (20 mg/mL × 50 mL = 1,000 mg) and remifentanil (20 mcg/mL × 50 mL = 1 mg). Controlled muscle relaxation was administered before the tracheal intubation, using 50 mg of rocuronium intravenously. The total time of anesthesia was 6 hours and 50 minutes. Anesthetic fade, which tends to increase the threshold of MEP, was not observed throughout the surgery.
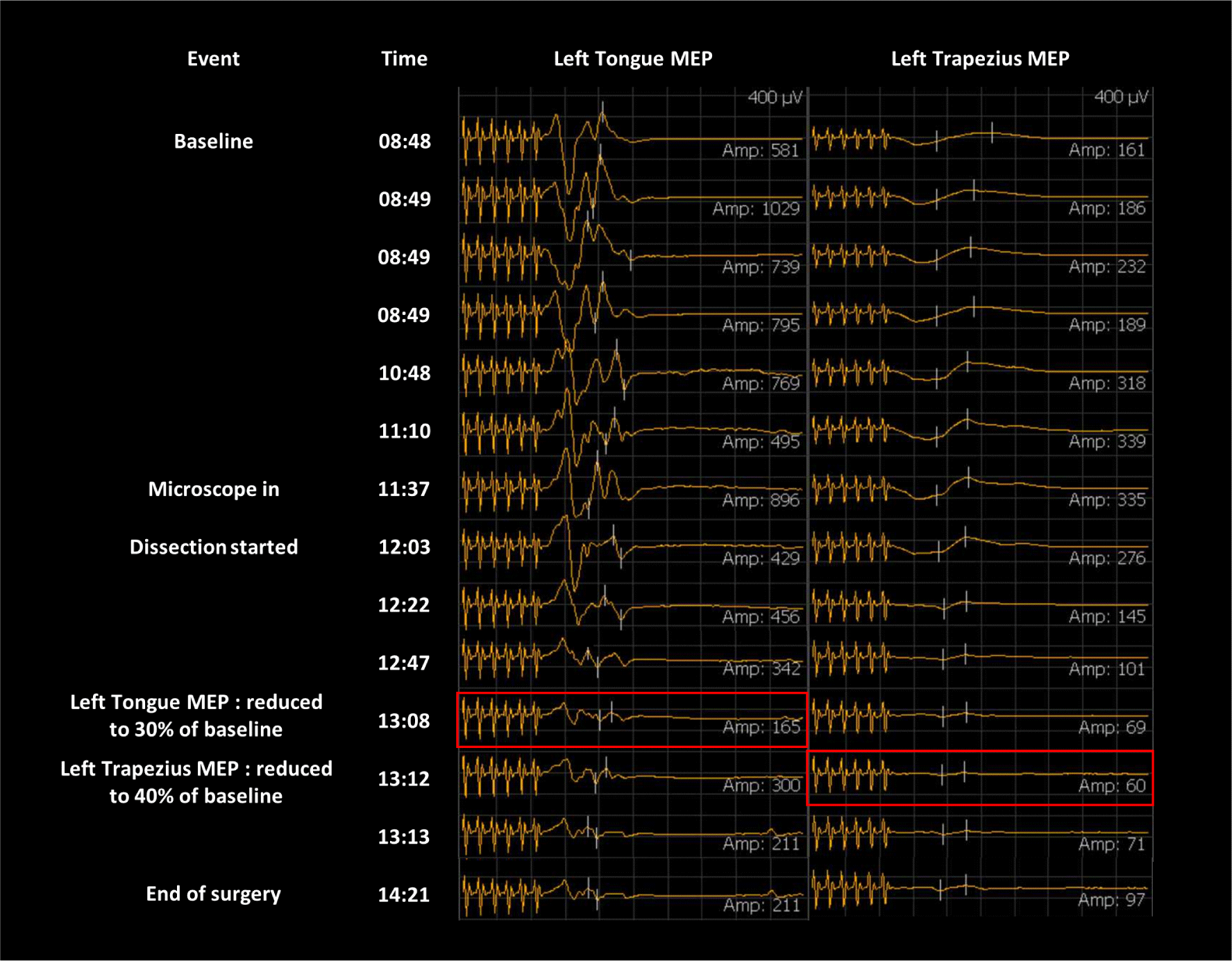
Signals of s-EMG were abundantly detected in the tongue muscle while removing a tumor attached to the branch of the left LCN. During the main procedure, the amplitude of the left tongue MEP was reduced to 30%, and left trapezius MEP was reduced to 40% of baseline, approximately 20 minutes after the s-EMG signal, and did not recover until the end of the operation (Fig. 2). Therefore, a part of the capsule wall of vestibular schwannoma was not able to be removed completely where severe adhesion existed between cerebellum and brainstem. There were no significant changes in facial MEP, SSEP, and BAEP during the surgery.
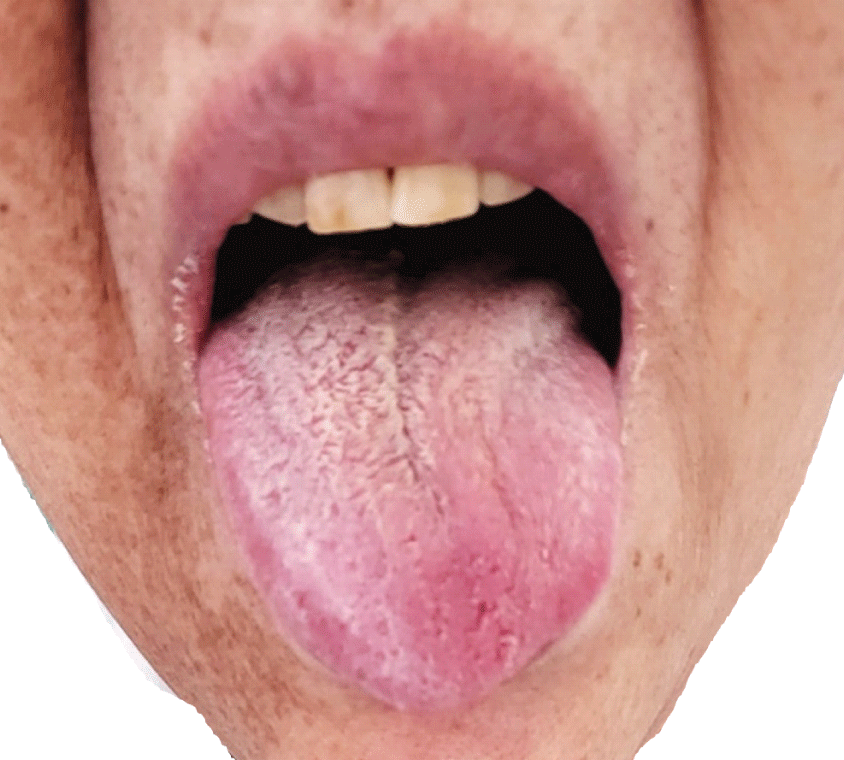
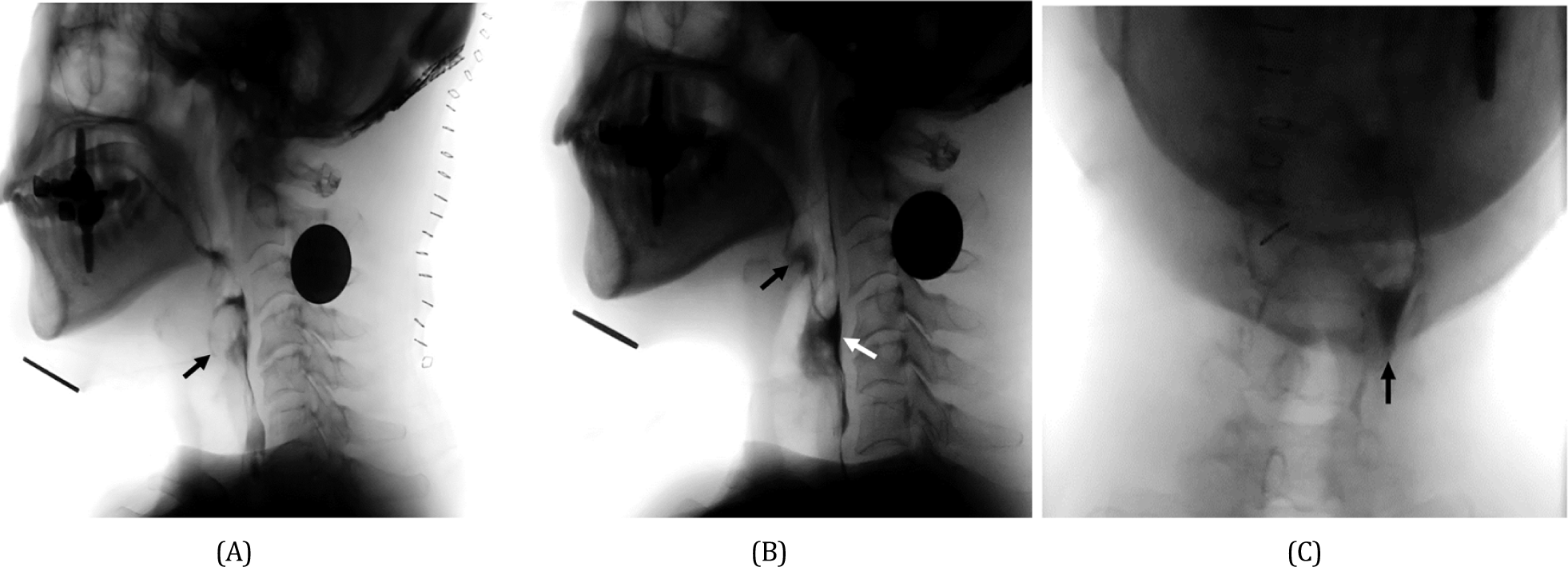
After the surgery, the patient complained of aggravated hoarseness and worsening dysphagia. The patient had difficulty initiating the swallowing reflex and had aspiration in all types of food. Therefore, a nasogastric tube was inserted immediately after the surgery. On physical examination, the tongue deviated to the left (Fig. 3) and the Medical Research Council scale for muscle strength of the left trapezius muscle was decreased to grade IV, weaker than the right side. Gag reflex was weak at the left side but not completely lost. However, the elevation of the soft palate was symmetric, and the deviation of the uvula was not definite. A videofluoroscopy swallowing study (VFSS) was performed two weeks after the surgery. The result demonstrated laryngeal aspiration in all kinds of modified diets (Fig. 4-A). Her swallowing reflex was markedly delayed because of severely impaired relaxation of the upper esophageal sphincter. Therefore, a large amount of residues remained in the vallecular and pyriform sinus after the swallow (Fig. 4-B), which further increases aspiration risk after a swallow. Also, this residue was mainly located on the left side (Fig. 4-C) as the left pharyngeal muscles were too weak to successfully continue pharyngeal constriction and swallow. On laryngoscopy, the left vocal fold was immobilized and the phonation gap was noted. The patient underwent an injection laryngoplasty into the left vocal fold one month after surgery. Hoarseness continued to improve after the injection laryngoplasty. Laryngeal EMG, performed 50 days after the surgery, revealed an electrophysiologic sign of peripheral cranial neuropathy of the left vagus nerve, accessory nerve, and hypoglossal nerve with abundant denervation potentials. Follow-up VFSS was conducted with the patient’s head rotated to the left side on the 4th day after injection laryngoplasty. The amount of post-swallow residue of the vallecular and pyriform sinus was partially improved. Deep penetration was detected in thin liquids, but there was no definite aspiration or penetration in a thick liquid such as yogurt and any solid food. Thus, the patient’s nasogastric tube could be removed, and a modified diet with thickening agents was provided to resume the oral diet. Two months after the surgery, the left deviation of the tongue and the weakness of the left trapezius were partially improved.
Discussion
We reported a case in which intraoperative ipsilateral trapezius and tongue MEP decrease was correlated with postoperative exacerbation of dysphagia, new onset of tongue deviation, and weakness of trapezius in surgery to remove cerebellopontine angle tumor including the brainstem. The tumor was suspected of involving LCN by MRI and preoperative symptom of dysphagia existed even before the surgery.
For IONM of transcranial MEP, orbicularis oculi, orbicularis oris, and trapezius were monitored bilaterally. However, tongue MEP was unilaterally monitored by inserting electrodes into the midline of the tongue. In this case, the cerebellopontine angle tumor was compressing only the left brainstem. For lesions involving the unilateral brainstem, significant results of tongue MEP which correlates with postoperative symptoms can be obtained even with unilateral monitoring. Bilateral monitoring is useful to compare amplitudes with the contralateral side as a control. However, corticobulbar MEP to tongue muscle elicits sufficiently high MEP amplitude [4], approximately 1.1 mV from a previous study and 1.0 mV at the baseline from this case. Compared to trapezius MEP, this well-demonstrated, high muscle MEP could suggest that even ipsilateral monitoring of CN XII is useful to monitor injury at LCN.
Reduction of trapezius and tongue MEP is a predictor of postoperative CN XI-XII deficit and subsequent symptoms of lower cranial neuropathy such as dysphagia and dysphonia. In this case, the amplitude of the left trapezius and tongue MEP decreased to 30% of the baseline, and dysphagia, tongue deviation, and trapezius weakness occurred right after the surgery. In a previous case series report showing changes in intraoperative tongue MEP [5], one patient had tongue MEP that disappeared during surgery but did not recover, and postoperative tongue deviation and fasciculation were developed. In the other patient, intraoperative tongue MEP was reduced by 50% and no sequelae occurred [5]. However, there is a paucity of studies that investigated the specific warning criterion for intraoperative MEP of LCN. Only one previous study, including 63 patients who underwent posterior fossa operation, investigated the predictive value of changes in the intraoperative MEPs of CN IX, X, and XII and postoperative functional outcomes of tongue or uvula deviation, implying a final-to-baseline ratio of MEP width could be a possible parameter with a cut-off of 1.03 ms [6]. As this case showed a possibility that the final MEP amplitude of the tongue might correlate with post-operative tongue and swallowing function, additional large-scale studies are necessary to develop more detailed criteria for changes of MEP of LCN that could be associated with postoperative cranial nerve dysfunction and dysphagia.
The limitation of this case is that for intraoperative LCN monitoring, only CN XI and CN XII were monitored, while CN IX and CN X were not included. It would have been helpful to perform free-running and triggered EMG in the stylopharyngeus muscles (CN IX) and vocal folds (CN X) [7] or to monitor laryngeal adductor reflex using endotracheal tube electrodes as well in the case of tumor near the brainstem [8]. However, transcranial MEP or EMG of CN IX and CN X requires distinct equipment or special attention to accurately place the needle electrode into the target muscle. Transcranial MEPs of CN XI and CN XII were selected preferentially because they are relatively more applicable targets. After all, insertion of the needle electrode is easy to perform even for novices and no special, expensive equipment is necessary.
In conclusion, intraoperative decrement of transcranial MEP in CN XI and CN XII can be a predictor of postoperative LCN dysfunction. IOMN for surgery of cerebellopontine angle tumor should include monitoring of LCN using transcranial MEP if the tumor is suspected to involve the brainstem or if the patient has preoperative symptoms of LCN dysfunction. For IOMN of CN XII, unilateral MEP monitoring at the midline of the tongue can effectively predict postoperative CN XII dysfunction especially if tumor is located on one side.







