Introduction
In CNS tumors, intramedullary tumors (IMTs) account for about 2%–4% [1]. The most common types are ependymoma and astrocytoma, followed by ganglioglioma and CNS lymphoma [2]. Tumor occurs most frequently in the order of cervical, thoracic, and lumbar, and this can lead to localized back or radicular pain, extremity weakness, spasticity, and urinary dysfunction. Some tumors, including the second most common astrocytoma, have a poor prognosis [3].
Although surgical resection, radiotherapy, chemotherapy are used for the treatment of IMTs, surgical removal is known to be the most effective regardless of the type of tumor [2]. Spinal cord has important tracts in small structures, and as well as symptoms caused by the tumor itself, sequelae from surgery can have impact on the quality of life of the patient. Therefore, it has emerged as an important issue to determine the exact tumor margin and to understand the positional relationship with the surrounding structures in advance. However, it is difficult to distinguish the corticospinal tract (CT) and dorsal column (DC) with the naked eye in the surgical field because the position of the anatomical landmarks usually distorted by the tumor.
To minimize injuries occur during surgery, a new method called spinal cord mapping was introduced, which differentiates CT and DC using evoked potential or D-wave [4,5]. However, the problem with previously devised methods for mapping is that when CT or DC are stimulated, both can induce a motor response, making it difficult to distinguish them [6]. Therefore, a method for mapping CT and DC has emerged using different recovery times of spinal cord interneurons targeted by CT and DC axons, which called double train paradigm [7]. This is the first domestic report of spinal cord surgery, which is double train method applied.
Case Presentation
A 54-year-old female patient visited the outpatient clinic complaining of lower back pain and left leg weakness. Symptoms have gotten worse over the past 2 months. Physical examination revealed decreased anal sphincter tone and 40% of sensory impairment on left lower extremity compared to normal side. Left lower extremity weakness showed grade IV- with spasticity, measured by the Medical Research Council (MRC).
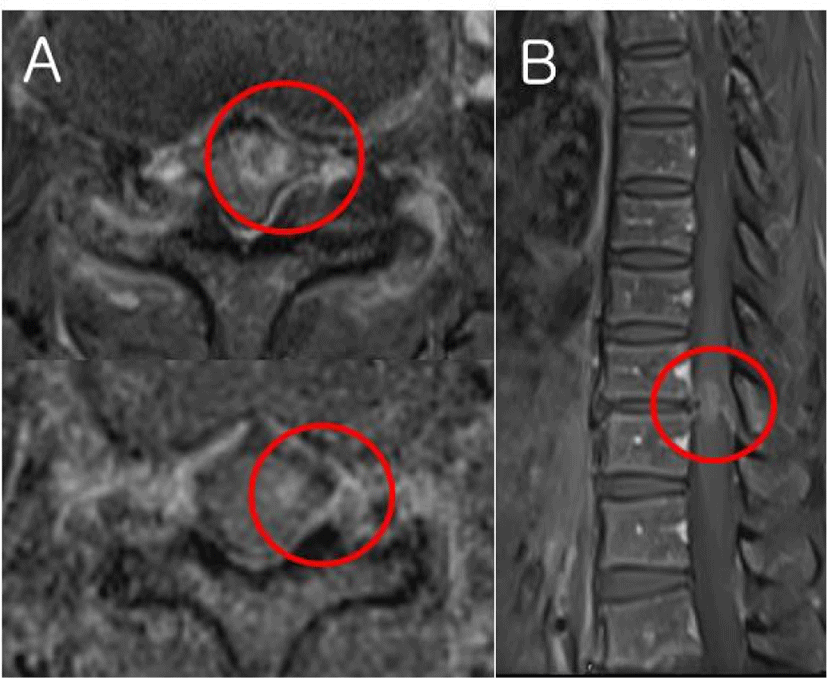
IMT on T10-11 was confirmed on spine magnetic resonance image (Fig. 1), and surgical removal was decided. Tumor was biased to the left, but there was a possibility that it invaded both the CT and the DC with a size of 24 mm. In addition to spinal cord mapping, motor evoked potentials (MEPs), sensory evoked potentials (SEPs), and bulbocavernosus reflex (BCR) with established interpretation and warning criteria were performed to minimize injury as intraoperative neurophysiologic monitoring. The D-wave could not be monitored because the waveform was not formed.
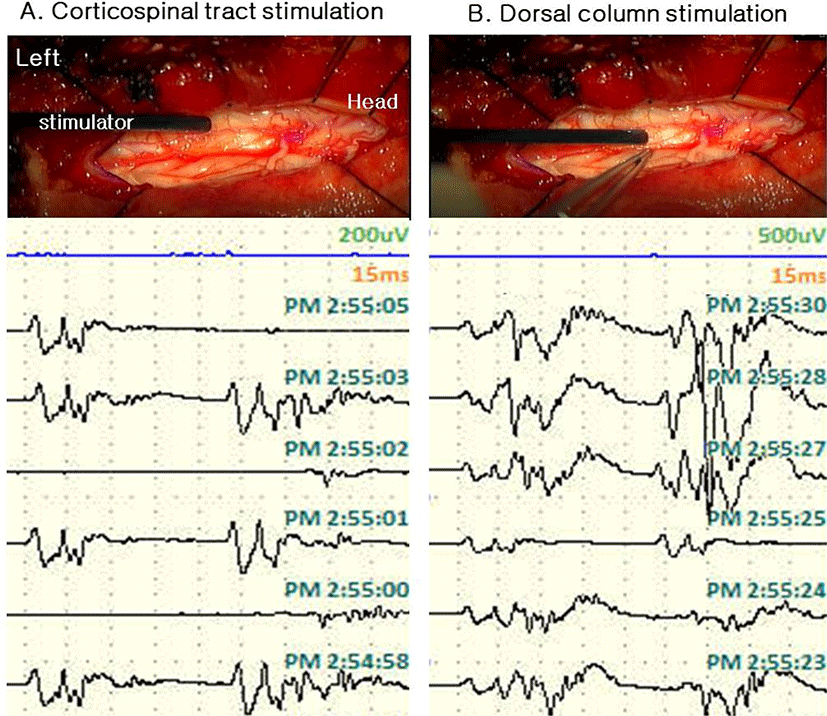
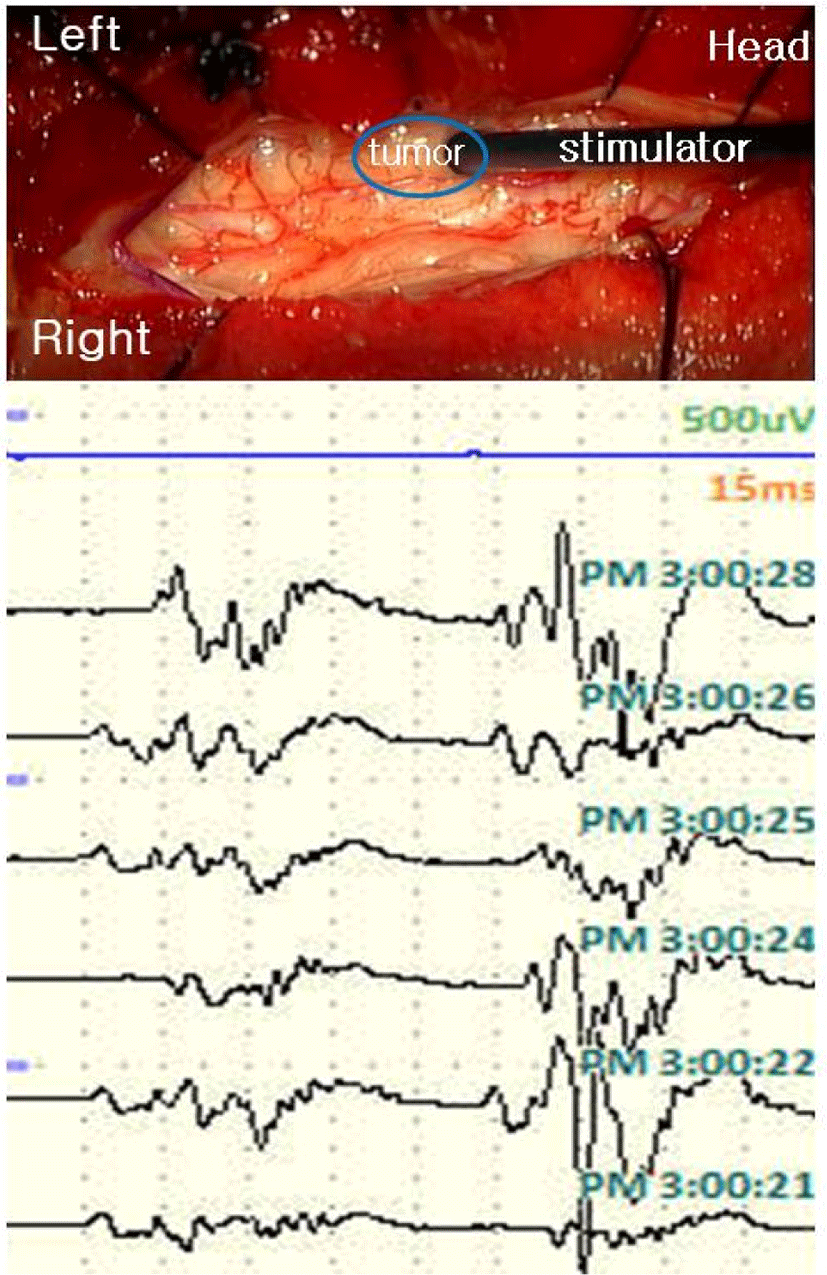
Spinal cord mapping was performed using a double train paradigm. Stimulation was given 5 times consecutively with 0.5 ms pulse duration from 0.5 mA to 5 mA with a bipolar stimulator, and responses were recorded from both lower extremity muscles [rectus femoris, tibialis anterior (TA), abductor halluces (AH)] and anal sphincter. The intertrain interval (ITI) was set to 60 ms. We started recording while stimulating the spinal cord, and before myelotomy, the expected waveform shape was confirmed from 0.5 mA. At the CT estimated by an anatomical landmark, the shape of the waveform was implemented equally at intervals of 60 ms. In the case of DC, the waveform was realized larger than the first waveform after 60 ms as expected in patient with spasticity. It was possible to distinguish between CT and DC even in the shape of the waveform for the stimulus, and the difference in the latency of the waveform for the first stimulus was also confirmed to be longer in DC (Fig. 2) However, in the vicinity of the tumor, the waveform was not well formed with relatively low intensity (0.5 mA to 3 mA). Also when we stimulated presumed CT location near the tumor at high intensity (4–5 mA), a waveform similar to that of DC was made, making it difficult to distinguish structures compared to normal tissues (Fig. 3). Stimulation was applied to each location to further check during tumor resection after myelotomy, but we terminated the examination with no recorded waveforms at all intensities.
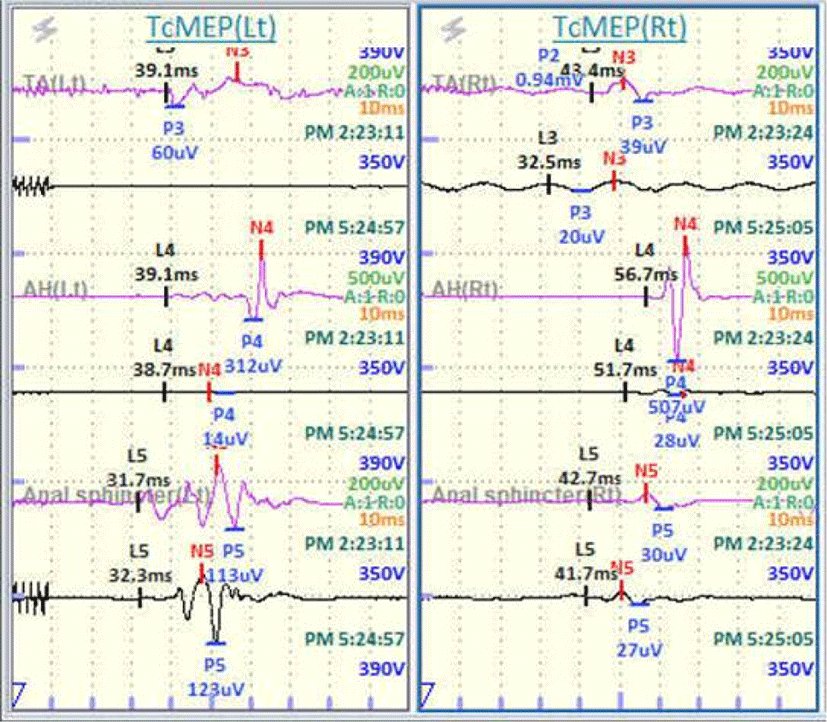
There were no significant deterioration in BCR during operation. However, the amplitudes of MEPs were reduced to 5% of the baseline in the bilateral TA and AH. No waveform was detected on bilateral posterior tibial SEPs from baseline (Fig. 4).
After surgery, the patient had motor weakness as MRC grade II, and the sensory deficit also worsened compared to before.
Discussion
For better surgical results, several spinal cord mapping methods have been devised to accurately identify the anatomy of the spinal cord during operation. Nevertheless, the previous mapping method had a clear limitation, in that it was difficult to distinguish the waveforms when CT and DC were stimulated. Reasons for this include not only the structure being too narrow, but also indirect and antidromic activation of the alpha motor neuron via a sensory reflex pathway collaterals in the DC [6].
We introduced double train stimulation to differentiate between the CT and the DC in spinal cord tumor resection. When stimulating the anatomically identified CT and DC of the patient, different morphology of the waves are recorded in the muscles. In CT, two waveforms with the same size and shape are made at intervals of time. When DC is stimulated, on the other side, the second wave is not formed, or a waveform with a larger amplitude is formed in patients with spasticity like this case [7]. The difference of waveform between these two stimulations is due to the recovery time of interneurons in the gray matter of the spinal cord of two pathways. We tested the ITI at 60 ms, because it takes about 70 ms for partial recovery and 150–300 ms for complete recovery in DC, unlike CT needs much shorter recovery time than 60 ms. Therefore, ITI at 60 ms could generate the same response after the second stimulation as the first stimulation only in CT [8]. The refractory time of DC, which is longer than CT could be attributed by several mechanism. But mainly, it occurs due to the polysynaptic end of DC at the alpha motorneurons of the anterior horn unlike the oligosynaptic end of CT [7]. Considering the recovery time it takes until the responses after second stimulation are the same as first stimulation in CT, not in DC.
In our case, the two structures were not well differentiated near the lesion, and the waveform of DC appeared when the location presumed CT was stimulated in high intensity. This is because the position of the normal structure was changed due to the tumor. Second, it is considered that a stronger stimulus was required than normal tissue for waveforms to appear, due to changes in surrounding tissues by tumor. Also, when stimulation applied to spinal cord with more than certain intensity, the structures are closely attached in a narrow place, both tracts may be stimulated together. Waveform was not generated after myelotomy at all, which made no practical help in accurately defining CT during tumor dissection. Therefore, it is necessary to check if there are any differences depending on the tumor size, location, and type, and a more precise stimulation method will be needed.
We confirmed that double train stimulation would be of great help in the mapping of spinal cord structures during surgery. However, more case experience and skills are needed for surgeon, neurologist, and technician to make a clear protocol.







